This site is no longer maintained and is presented for archive purposes only
| D-values (decimal reduction time; the time required to kill 1 log concentration of bacteria) were determined for both human and bovine strains (Dominic, Ben, BO45, and ATCC 19698) of Mycobacterium paratuberculosis in 50 mM lactate solution (pH 6.8) and in milk at four temperatures (62, 65, 68, and 71oC). Viable M. paratuberculosis organisms were quantified by a radiometric culture method (BACTEC). Thermal death curves for the M. paratuberculosis strains tested were generally linear, with R2 of >= 0.90, but a few curves (R2, 0.80 to 0.90) were better described by a quadratic equation. The human strains (Dominic and Ben) had similar D-values in milk and in lactate solution. However, D-values for the bovine strains (BO45 and ATCC 19698) were significantly different depending on the menstruum. D-values for low-passage clinical strains (Dominic, Ben, and BO45) were lower than those of the high-passage laboratory strain (ATCC 19698). The D-value based on pooled data for clinical strains of M. paratuberculosis in milk at 71oC (D71oC) was 11.67 s. Pooled D62oC, D65oC, and D68oC of clinical M. paratuberculosis strains in milk were 228.8, 47.8, and 21.8 s, respectively. The Z value (the temperature required for the decimal reduction time to traverse 1 log cycle) of clinical strains in milk was 7.11oC. The D-values of clumped and single M. paratuberculosis cells were not significantly different. The D-values of all M. paratuberculosis strains tested were considerably higher than those published for Listeria, Salmonella, and Coxiella spp. and estimated for Mycobacterium bovis, indicating that M. paratuberculosis is more thermally tolerant. This study supports the premise that M. paratuberculosis may survive high-temperature, short-time pasteurization when the initial organism concentration is greater than 101 cells/ml. |
Both Crohn’s disease (a human illness of unknown etiology) and Johne’s disease (an animal disease caused by Mycobacterium paratuberculosis) are chronic, incurable, inflammatory bowel diseases. Their symptoms are similar: chronic diarrhea and weight loss33. In the 1980s and early 1990s, investigators reported the possible etiological role of M. paratuberculosis in Crohn’s disease after isolating this organism from Crohn’s patient tissues (intestines and lymph nodes) and detecting a DNA insertion sequence (IS900) unique to M. paratuberculosis 3,5-7,9,14,17-19,23-25,27.
Milk has been proposed as one possible source of M. paratuberculosis infection. The organism has been isolated from raw milk29,31,32, and IS900-positive acid-fast bacteria have been recovered in broth cultures of retail pasteurized milk22. Taylor et al.32 and Sweeney et al.31 reported that M. paratuberculosis was cultured from 9 of 26 milk samples (35%) obtained from cows with clinically advanced Johne’s disease, as well as 9 of 77 milk samples (11.6%) obtained from infected but clinically normal cows. Streeter et al.29 also isolated M. paratuberculosis from 2.4% of milk samples taken from 126 clinically normal cows. From 1991 to 1993, 312 commercially pasteurized bovine whole milk samples were randomly obtained from retail outlets in England. Nine of 18 M. paratuberculosis PCR-positive milk samples (50%) and 6 of 36 PCR-negative milk samples (16%) yielded broth cultures of acid-fast bacteria that were identified as M. paratuberculosis by IS900 PCR22.
In 1993, Chiodini and Hermon-Taylor4 demonstrated that bovine and human strains of M. paratuberculosis survive pasteurization and that human strains are generally more heat resistant at 72oC than are bovine strains. Grant et al.15 showed that M. paratuberculosis strains were not inactivated by low-temperature holding (LTH) (63.5oC for 30 min) or high-temperature, short-time (HTST) (71.7oC for 15 s) pasteurization methods. However, neither study determined the D-value (decimal reduction time; the time required to kill 1 log concentration of bacteria) or Z value (the temperature required for the decimal reduction time to traverse 1 log cycle) for M. paratuberculosis. Measurement of the D-value is important for assessing the ability of thermal processing techniques in food manufacturing to kill this potential pathogen. Thus, the objective of this study was to measure D-values for M. paratuberculosis.
Bacterial strains. Mycobacterium avium 6317, isolated in our lab from a bird with avian tuberculosis, and Listeria monocytogenes Scott A, obtained from Charles W. Kaspar, Food Research Institute, University of Wisconsin-Madison, Madison, Wis., were used as thermal-tolerance reference strains. M. avium 6317 was cultured in 7H9 broth medium containing 10% (vol/vol) Middlebrook OADC (oleic acid, dextrose, catalase; Difco, Detroit, Mich.) and (0.5% (vol/vol) Tween 80 (Sigma, St. Louis, Mo.) for 2 weeks at 37oC. Cells were pelleted by centrifugation at 10,000 X g for 20 min. The cell pellet was then homogenized with an overhead stirrer (Wheaton Instruments, Milville, N.J.) for 4 min on ice and used as the inoculum.
L. monocytogenes Scott A was inoculated into a 100-ml Erlenmeyer flask containing 30 ml of Trypticase soy-0.6% (wt/vol) yeast extract (TSYE) broth (Difco) and was incubated at 37oC for 48 h with shaking (200 rpm). The culture was diluted with TSYE broth to an optical density at 600 nm of 0.2. The diluted culture was suspended in 50 mM lactate solution (pH 6.8) to test thermal tolerance at 62oC. The final concentration was approximately 106 CFU/ml.
Two bovine strains (ATCC 19698 and BO45) and two human strains (Dominic and Ben [provided by R. J. Chiodini, Rehoboth, Mass.]) of M. paratuberculosis were used. The bovine strains were cultured in 100 ml of 7H9 broth medium containing 10% (vol/vol) Middlebrook OADC (Difco), 0.5% (vol/vol) Tween 80 (Sigma), and 0.0002% (wt/vol) mycobactin J (Allied Monitor Inc., Fayette, Mo.) for 4 months. The human strains were cultured similarly but without Tween 80. M. paratuberculosis cultures were centrifuged at 15,000 X g for 30 min, and the cell pellets were suspended in 30 ml of 7H9 broth and homogenized with an overhead stirrer (Wheaton Instruments) for 4 min on ice to break up large clumps of M. paratuberculosis cells. As a result, the suspensions were predominantly comprised of small clumps and single cells. The homogenized cell pellet suspensions were stored at -70oC. The identities of all M. paratuberculosis strains were verified by testing for IS900 both before and after heat treatment.
Menstruums. Lactate solution and milk were used as menstruums for heat treatment. Lactic acid (0.2 M) was adjusted with 0.2 M NaOH to pH 6.8. The pH was monitored throughout the study and sustained within ±0.1 pH unit. The final concentration of the lactate solution used as a menstruum was 50 mM. Raw milk was collected from healthy Holstein cows by hand milking into sterilized plastic bottles after the teats were cleaned and disinfected with 70% (vol/vol) ethanol. If the raw milk was not immediately used, it was stored in a refrigerator for no more than 2 days. Before use as menstruums in the thermal tolerance study, lactate solution and raw milk were preheated to the target temperature for 30 min.
Heat treatment. After preheating the menstruum for 30 min in a water bath (5L-M; Fisher Scientific Co., Medford, Mass.) adjusted to the reaction temperature (62, 65, 68, or 71oC), test bacteria were suspended in a total volume of 1.5 ml of preheated menstruum in Wheaton vials (12 by 35 mm; Kimble Glass Co., Vineland, N.J.). Each vial was scaled and immersed in the water bath. The menstruum in the vials was kept 4 cm below the water level in the bath. The temperature was monitored at all times with a mercury-filled thermometer (Fisher Scientific Co.) and was maintained within ±0.5oC. The final concentration of bacteria was 105 to 106 cells/ml. After being heated for various time intervals, the vials were removed from the water bath and immediately chilled by immersion in ice water. The menstruum from each vial was then inoculated into bacteriologic culture medium as part of the enumeration method described below.
Enumeration methods. Viable M. paratuberculosis cell numbers were estimated by a radiometric culture method (BACTEC) in triplicate. Three Wheaton vials and three BACTEC bottles were used for each heating interval. The heat-treated menstruum containing 105 to 106 cells/ml was inoculated into commercial BACTEC 12B bottles (Becton Dickinson Microbiologic Systems, Sparks, Md.) containing 1.0 ml of egg yolk suspension (Difco), 0.1 ml of mycobactin J solution (40 µg/ml), and 0.1 ml of an antibiotic cocktail containing vancomycin, amphotericin B, and nalidixic acid. The final concentrations of these antibiotics in the radiometric broth (BACTEC 12B bottles) were 8.4, 16.8, and 25.2 µg/ml, respectively. The bottles were incubated at 37oC without agitation and read on a BACTEC 460 instrument without CO2 daily for 45 days. The BACTEC 460 instrument measured 14CO2 gas produced by metabolism of [14C]palmitate in the medium. From the total amount of 14CO2 gas produced (cumulative growth index), the number of M. paratubercolosis cells was estimated by comparison to a standard growth model described by the following equation16:
| _ | ln [Ym/(Y - 1)] - ln (BCt0Dt20Et30) | |
| X | = | _______________________________ |
| ln (Ct1Dt21Et31) |
where Y is the cumulative growth response in units of 14CO2 released; Ym is a fixed value bounded by the maximum cumulative growth response, which is 12,950; X is the inoculum size; t is the incubation time; and B through E are regression coefficient constants determined to have the following values: B = 10,340, C0 = 1.2217, C1 = 0.84345, D0 = 0.98959, D1 = 1.004644, E0 = 1.00008339, and E1 = 0.99996559.
Viable bacterial cell counts determined by radiometric culture were compared to conventional plate counts after heat treatment of M. paratuberculosis ATCC 19698 in lactate solution at 62oC. The heat-treated menstruum was serially diluted and inoculated onto 7H9 agar medium containing 10% (vol/vol) Middlebrook OADC (Difco) and 0.0002% (wt/vol) mycobactin J (Allied Monitor Inc.). The 7H9 agar medium was incubated for 8 months at 37oC, at which time the colonies were counted.
Enumeration of viable M. avium 6317 cells was done by the radiometric culture (BACTEC) method without mycobactin J and egg yolk solution. The numbers of viable M. avium cells were estimated by comparison to a standard curve with the same growth model as that used for M. paratuberculosis but with different coefficients8.
L. monocytogenes Scott A was serially diluted in lactate solution and plated on TSYE agar. The plates were incubated for 4 days at 37oC, and the colonies were counted.
D-values, Z values, and the pasteurization line. D-values were calculated from the slope of the best-fit line graphically determined by plotting the log10 of M. paratuberculosis survivors per milliliter versus the time of heat exposure at each reaction temperature30. Z values were determined by plotting the log10 of D-values versus the reaction temperatures. The pasteurization line, i.e., the time-temperature requirements to completely inactivate all viable cells34, was estimated by regressing the estimated time to kill 100% of the target organisms based on best-fit regression lines from thermal death curves. The best-fit lines were calculated by Minitab regression analysis software (MINITAB Inc., State College, Pa.).
Reproducibility of D values. D-value reproducibility was evaluated in two heat treatment trials conducted with M. paratuberculosis ATCC 19698 at all target temperatures. Each heat treatment trial was independent. Viable M. paratuberculosis cells were counted by the radiometric method.
Effect of clumping on D-values. M. paratuberculosis Ben was cultured in 100 ml of 7H9 culture broth supplemented with mycobactin J and Middlebrook OADC but without Tween 80 for 4 months at 37oC. The culture broth was centrifuged at 15,000 X g for 30 min at 4oC, and the cell pellet was suspended in 30 ml of 7H9 broth. A portion of the pellet suspension was stored at -70oC to be used as clumped M. paratuberculosis cell samples. The remainder of the pellet was resuspended in 7H9 broth and homogenized with an overhead stirrer (Wheaton Instruments) for 4 min on ice and then sequentially filtered through 5.0-, 3.0-, and l.2-µm-pore-size polycarbonate filters. The homogenized and filtered suspension was used for single-cell M. pamiubereolosis samples. D values for the clumped and single-cell samples were measured as described above.
Statistical analysis. Linear regressions of thermal death curves were conducted to determine D-values for M. paratuberculosis based on the concepts presented by Chatterjee and Price2 and by Draper and Smith12. Differences among the slopes of thermal death curves were analyzed by using the SAS program (release 6.10; SAS Institute, Inc., Cary, N.C.). P values of <0.05 were considered significant.
Reproducibility of D-values. D-values and Z values of M. paratuberculosis ATCC 19698 were calculated from two heat treatment trials in lactate solution (50 mM, pH 6.8). The D-value 62oC (D62oC), D65oC, D68oC and D71oC for M. paratuberculosis ATCC 19698 from the first trial were 324.6, 55.7, 23.8, and 13.0 s, respectively; those from the second trial were 266.2, 52.5, 23.6, and 10.6 s, respectively. The Z values of the first and second trials were 6.57oC and 6.67oC, respectively.
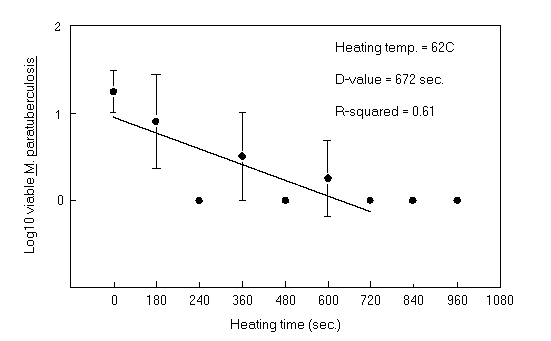
Comparison of radiometric culture with plate counts in the quantification of M. paratuberculosis. M. paratuberculosis ATCC 19698 was heat treated in lactate solution at 62oC, and samples taken at each time interval were cultured for 8 months on 7H9 agar plate medium. Aliquots of the same heat-treated strain were inoculated into radiometric (BACTEC) bottles. The number of viable M. paratuberculosis cells obtained by standard plate counting was lower than that determined radiometrically. There was a large standard error for each plate count, and the thermal death curve based on plate counting was not as linear (R2 = 0.61). D62oC calculated from plate count results was 672 s (Fig. 1). Thermal death curves were linear (R2 = 0.95) when viable counts were determined by radiometric culture, and D 62oC was lower (324.6 s).
Effects of clumping on thermal tolerance. The unfiltered culture suspension of strain Ben was comprised of single cells and small and large clumps of M. paratuberculosis (Fig. 2A). After homogenization and filtration of the culture, the large clumps were eliminated and the suspension was comprised only of single cells and small clumps of M. paratuberculosis (Fig. 2B). Thermal death curves of unfiltered (clumped) and filtered (single-cell) M. paratuberculosis Ben in lactate solution at 65oC were obtained (data not shown). D65oC of the single-cell sample (38.2 s) and the clumped sample (52.9 s) were not significantly different (P = 0.0754).
D-values in lactate solution. The thermal death curve of L. monocytogenes Scott A at 62oC was linear (R2 = 0.99) with narrow 95% confidence intervals, and D62oC was 52.3 s (data not shown).
The thermal death curves of M. avium 6317 in lactate solution were best described by a quadratic function (data not shown). However, by linear regression R2 values were >0.90 at all temperatures tested. D 62oC, D 65oC, D68oC, and D71oC for M. avium 6317 in lactate solution were 77.7 s (R2 = 0.95), 31.6 s (R2 = 0.96), 14.9 s (R2 = 0.93), and 9.5 s (R2 = 0.91), respectively. The Z value of strain 6317 was 9.79oC, with R2 of 0.98.
Regression analysis results with 95% confidence intervals for M. paratuberculosis Dominic in lactate solution are shown in Fig. 3 and are representative of results for all M. paratuberculosis strains tested. At 62oC and 65oC, the thermal death curves were linear (R2, 0.92 and 0.98, respectively). At higher temperatures, 68 and 71oC, the curves were less linear (R2, 0.83 and 0.84, respectively) and better described by quadratic functions. The D-values, however, determined from the slope of linear regression curves for M. paratuberculosis Dominic were 139.3, 45.5, 21.3, and 13.1 s at 62, 65, 68, and 71oC, respectively. From these D-values, a Z value of 8.8oC was calculated for strain Dominic.
The D-values of the three other M. paratuberculosis strains tested in 50 mM lactate solution (pH 6.8) were also calculated after linear regression analysis (Table 1). The D-values for ATCC 19698 and BO45 at 62oC were significantly different (P = 0.001), but at 65, 68, and 71oC, the D-values for these two bovine strains of M. paratuberculosis were not significantly different (P> 0.1). The D-values for the two human strains tested were the same at each temperature tested in lactate solution (P> 0.1).
D-values of M. paratuberculosis in milk. Regression analysis of thermal death curves for M. paratuberculosis strains suspended in milk were performed. Although quadratic patterns of the inactivation were evident, linear regression was performed, giving R2 values of > 0.90 at all test temperatures. D62oC, D65oC, D68oC, and D71oC for strain Dominic were 162.5, 38.9, 20.2, and 12.3 s, respectively (Fig. 4). A Z value of 8.24oC was determined from these values.
Table 2 shows the D-values and Z values of all M. paratuberculosis strains tested in milk. The D-values of the human strains were the same at every temperature in the milk menstruum (P > 0.1). However, the D-value differences between the bovine strains (ATCC 19698 and BO45) at 62 and 65oC were significant (P, 0.0160 and 0.0075, respectively).
One hundred percent killing time for M. paratuberculosis. Linear regression analysis was used to estimate the time to achieve 100% killing of 106 cells/ml for M. paratuberculosis Dominic at 62, 65, 68, and 71oC. Times of 834, 276, 132, and 78 s in lactate solution and 978, 234, 120, and 72 s in milk were determined for the respective temperatures. Table 3 shows the estimated 100% killing times for pooled data for clinical M. paratuberculosis strains (Dominic, Ben, and BO45) when the initial cell populations were 106, 105, 104, 103, 102, and 101 organisms/ml.
Methods for determination of thermal inactivation rates for bacteria have been thoroughly reviewed by Donnelly et al.11, who measured the thermal resistance of L. monocytogenes by the sealed-tube method and the test tube method. The sealed-tube method, in which 2-ml glass reaction vials containing 1.5 ml of whole milk are crimp sealed with metal caps and heated in a water bath which has been maintained at 62oC, produced linear death curves. However, the test tube method, in which test tubes containing 10 ml of menstruum were placed in a water bath, yielded nonlinear death curves and showed that L. monocytogenes survived after 30 min of heating at 62, 72, 82, and 92oC. They concluded that the sealed-tube method was more accurate. Thus, this method was selected for the present study.
To validate our technique, the D-value of L. monocytogenes Scott A at 62oC was determined. The thermal death curve was linear, and the D62oC of 52.3 s was similar to the D62oC of L. monocytogenes strains reported by Donnelly et al. (D62.7oC = 54 s)10. Therefore, we concluded that our method of D-value determination was consistent with other studies.
Preheating of the menstruum significantly altered D-value determinations for M. paratuberculosis (data not shown). Without preheating of raw milk at 71oC, M. paratuberculosis ATCC 19698 was not inactivated after 90 s of heating and showed only a 1-log10 reduction in viable organisms. However, with preheating of the menstruum, 106 cells/ml were completely inactivated after 90 s of heating at 71oC.
At higher test temperatures, thermal death curves for M paratuberculosis tended to be curvilinear and best described by quadratic rather than linear functions. However, when linear regression was applied to these data, R2 values were invariably >0.90. Thus, linear regression was deemed appropriate for D-value determination. Use of linear regression was a more statistically conservative approach to comparison of D-values and also insured that the method of data analysis was consistent with thermal tolerance studies focusing on other bacterial pathogens.
We compared D-values between two independent heat treatment trials with M. paratuberculosis ATCC 19698 in lactate solution to verify the reproducibility of the thermal inactivation experiments. Both trials gave the same D-value estimates at all four temperatures tested (P = 0.3996), supporting our conclusion that the method for determination of the thermal inactivation rate was reproducible.
Thermal inactivation results based on two different methods for quantification of M. paratuberculosis cells surviving heat treatment-radiometric and standard plate counting-were compared by using strain ATCC 19698 suspended in lactate solution at 62oC as a model system. Since thermally injured cells may require a longer time to grow than uninjured cells, plates inoculated with heat-treated M paratuberculosis ATCC 19698 were incubated for 8 months. When viable M. paratuberculosis cell counts were determined by plate counting, the thermal death curve was not linear (R2 = 0.61) and had a high D-value (672 s). Large colonies (about 5 mm in diameter) were observed on 7H9 agar plates, which may have resulted from deposition of large clumps of M. paratuberculosis on the plates. By radiometric quantification, the thermal death curves were linear, showing a gradual reduction in the population of M. paratuberculosis organisms with increasing heating time. Schroederus28 demonstrated that radiometric quantification could estimate the actual number of M. paratuberculosis cells regardless of whether mycobacteria were in clumps or existing as single cells. This suggests that standard plate counting methods are not as accurate as radiometric counts for thermal tolerance studies of M. paratuberculosis because of the strong influence of clumping on viable cell counts.
Grant et al.15 obtained a concave thermal death curve at 63.5oC, showing rapid death at 10 min of heating and slow death, or "tailing," after 10 min of heating. They considered the tailing to be due to clumped cells. In the present study, the thermal death curve of clumped M. paratuberculosis cells was linear and did not produce tailing by radiometric counting methods. The D65oC of clumped M. paratuberculosis was not significantly different from that of single M. paratuberculosis cells (P = 0.0754). However, there appeared to be a trend for faster inactivation of the single-cell samples (240 s for single cells versus 300 s for clumped cells) when the initial concentrations of M. paratuberculosis in both samples were 106 cells/ml.
Chiodini and Hermon-Taylor3 reported that human strains of M paratuberculosis had more thermal tolerance at 63oC and 72oC in whole milk than did bovine strains. However, in our study, a bovine strain (M. paratuberculosis ATCC 19698) showed more thermal tolerance than both human strains, as well as the clinical bovine strain BO45, for most menstruum/temperature combinations.
The influence of menstruum on D-value was largely strain dependent. D-values for M. paratuberculosis ATCC 19698 were significantly different in lactate solution versus milk at 62, 65, and 71oC (P, 0.0025, 0.0311, and 0.0392, respectively). D-values for strain BO45 in milk versus lactate solution were different only at 62oC (P = 0.0008). Thermal death curves of human strains were the same regardless of the type of menstruum (P > 0.05).
Strain differences may be explained by changes induced in M. paratuberculosis by in vitro cultivation. Strain ATCC 19698 is a high-passage reference strain21, while strain BO45 is a relatively low-passage strain isolated in our lab from a cow with Johne’s disease. The human strains are also relatively low-passage clinical isolates5-7. Thus, we hypothesize that the differences in D-values among M. paratuberculosis strains are primarily dependent upon the level of passage. To verify this hypothesis, we combined the thermal inactivation data of the human strains and compared the regression slope with that of each bovine strain. The D-values of strain ATCC 19698 and of the human strains derived from pooled data were significantly different at 62, 65, and 71oC in lactate solution (P, 0.0051, 0.0170, and 0.0169, respectively) and at 65 and 71oC in milk (P, 0.0002 and 0.0451, respectively). However, the D-values for the low-passage bovine strain, BO45, and for the human strains derived from pooled data were not significantly different in lactate solution or milk (P > 0.1) except at 62oC (P = 0.0112). Hence, the low-passage M. paratuberculosis clinical strains showed the same thermal tolerance patterns in both milk and lactate solution and were more sensitive to killing by heat than the high-passage strain.
Because thermal tolerance results for the M. paratuberculosis human strains and the clinical bovine strain BO45 were not significantly different, we pooled the thermal inactivation data to establish D-values reflecting most strains of M. paratuberculosis. D-values of the clinical strains (BO45, Dominic, and Ben) were 228.8, 47.8, 21.8, and 11.7 s at 62, 65, 68, and 71oC, in milk. In lactate solution, D62oC, D65oC, D68oC and D71oC were 179.2, 44.2, 19.4, and 12.2 s, respectively. Based on the pooled data, the estimated 100% killing time was 1,374, 288, 132, and 72 s at 62, 65, 68, and 71oC, respectively, in milk when the initial concentration of M. paratuberculosis was 106 cells/ml. With estimated D-values for clinical strains of M. paratuberculosis, Table 3 shows the estimated 100% killing times of M. paratuberculosis for initial cell concentrations of 106, 105, 104, 103, 102, and 101 cells/ml. The data indicate that M. paratuberculosis can be controlled by the current HTST pasteurization temperature-time combination if there are <=101 organisms/ml in milk.
There has been a long scientific history of assessing the thermal resistance characteristics of bacteria. Pasteurization of milk was established in the early 1900s for the purpose of killing Mycobacterium bovis, considered then to be the most heat-resistant human pathogen associated with milk34. In 1957, the recommended LTH pasteurization temperature was increased from 61.7 to 62.8oC to insure effective killing of approximately 106 Coxiella bumetii cells/ml, the cause of Q fever in humans. HTST pasteurization (15 s at 71.7oC) requirements were not changed, however, as that time-temperature combination was sufficient to kill both C. burnetii and M. bovis based on laboratory studies done with roughly 105 to 106 organisms/ml of raw milk13,34.
M. bovis has been reported to be less thermally tolerant than other mycobacteria26. Merkal et al.20 found that M. bovis strains were inactivated at a temperature 6 to 7oC lower than that necessary to inactivate M. avium. Our study confirms the findings of Merkal et al.: M. avium 6317 in lactate solution had a higher D-value (D71oC = 9.5 s) than that of M. bovis (D71.7oC = 4 s) estimated in previous reports26,34. When we then compared M. avium with M. paratuberculosis, we found that the D-values of M. avium 6317 were significantly lower than those of the combined M. paratuberculosis clinical strains and ATCC 19698 at every temperature tested (P < 0.03). Therefore, of these three mycobacteria, the microorganism used to set pasteurization standards, M. bovis, is the most sensitive to heat, followed by M. avium and then M. paratuberculosis.
Chiodini and Hermon-Taylor4 reported in 1993 that 5 to 9% of bovine M. paratuberculosis cells survived heat treatment at 63oC for 30 min and that 3 to 5% survived after heat treatment at 72oC for 15 s. In 1996, Grant et al.15 supported Chiodini’s findings by isolating this acid-fast organism from spiked milk samples after LTH and HTST pasteurization treatment. They isolated M. paratuberculosis from 27 of 28 (96%) and 29 of 34 (85%) spiked milk samples (106 to 107 CFU/ml) heat treated by the LTH and HTST methods, respectively. M. paratuberculosis was also recovered from 14 of 28 (50%) and 19 of 33 (58%) milk samples treated by the LTH and HTST methods, respectively, when the initial concentration of organisms in milk was 103 to 104 CFU/ml. Our results support the work of Chiodini and Hermon-Taylor4 and of Grant et al.15. We conclude that both human and bovine strains of M. paratuberculosis may survive the HTST pasteurization method when the initial M. paratuberculosis concentration is greater than 101 organisms/ml (Table 3).
Sweeney et al.31 found low concentrations of M. paratuberculosis in milk samples collected from asymptomatic cows infected with M. paratuberculosis (2 to 8 CFU per 50 ml of sample). Grant et al.15 suggested that the concentration of M. paratuberculosis in milk could be as high as 104 CFU/ml due to the potential for fecal contamination of milk during collection: However, in order to provide a margin of safety above the upper limit of M. paratuberculosis potentially prevailing in natural milk, we assumed the concentration of M. paratuberculosis in milk to be 2 logs higher (106 organisms/ml). Pasteurization lines for the clinical M. paratuberculosis strains at 106 organisms/ml were estimated and compared with pasteurization lines calculated from a previous study in which comparable concentrations of target bacteria were used13,34 (Fig. 5). This analysis indicates that M. paratuberculosis is more thermally tolerant than M. bovis and C. burnetii and could potentially survive HTST pasteurization but not LTH pasteurization.
In summary, D-values of human and bovine M. paratuberculosis strains were obtained by the sealed-tube method. Although a quadratic function was observed in some thermal death curves for M. paratuberculosis, most thermal death curves were linear. D-values for M. paratuberculosis ATCC 19698 were higher than for other M. paratuberculosis strains tested, and D-values measured in milk were higher than in lactate solution. D-values for low-passage clinical strains were not statistically different from each other, and D-values of clumped and single M. paratuberculosis cells were not significantly different. Based on the pooled data for all clinical strains and the D-values estimated under our laboratory conditions, the M. paratuberculosis strains tested showed the potential to survive HTST pasteurization methods if the initial number of M. paratuberculosis cells is >101 organisms/ml of milk. These findings are consistent with a recent report of the detection of M. paratuberculosis in long-term cultures of retail milk samples in England22.
We express our gratitude to Charles W. Kaspar, Food Research Institute, University of Wisconsin~Madison, Madison, Wis., for valuable advice and critical review of the manuscript. We thank R. J. Chiodini, Rehoboth, Mass., for providing M. paratuberculosis human strains. We also thank Sorab Kim and Murray Clayton, Department of Statistics, University of Wisconsin-Madison, for help with statistical analysis.
This research was funded in part by the Wisconsin Milk Marketing Board (project UW 9507).

FIG. 1. Thermal death curve for M. paratuberculosis ATCC 19698 in lactate solution at 62oC enumerated by the standard plate counting method. Each point represents the mean of replicate plates with standard error bars; the linear regression line is indicated.
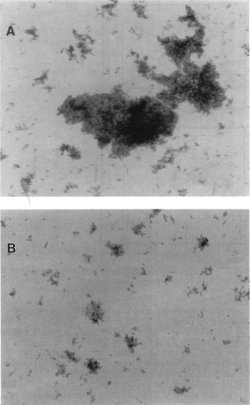
FIG. 2. Clumped (A) and single (B) M. paratuberculosis Ben cells. Both samples were acid-fast stained, and pictures were taken with a light microscope (Zeiss, Oberkochen, Germany). Magnification, X8,300.
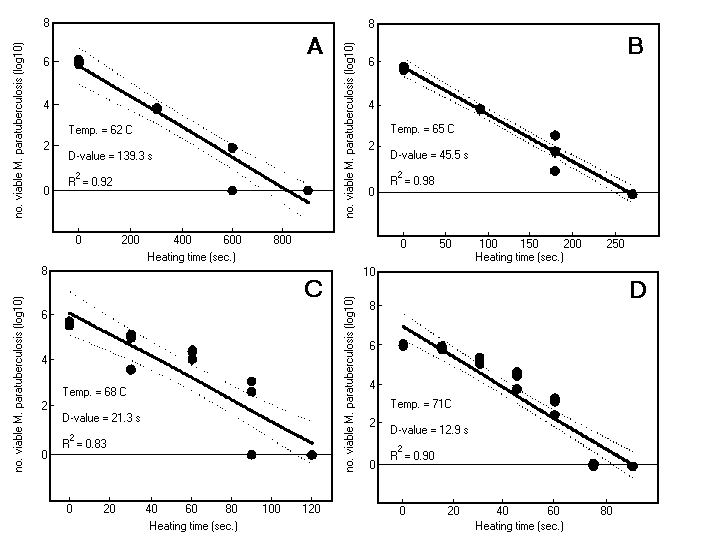
FIG. 3. Thermal death curves for M. paratuberculosis Dominic in lactate solution. (A) 62oC. (B) 65oC. (C) 68oC. (D) 71oC. Data points are log10 counts of M. paratuberculosis cells. Thick solid line, linear regression line; dotted lines, 95% confidence intervals.
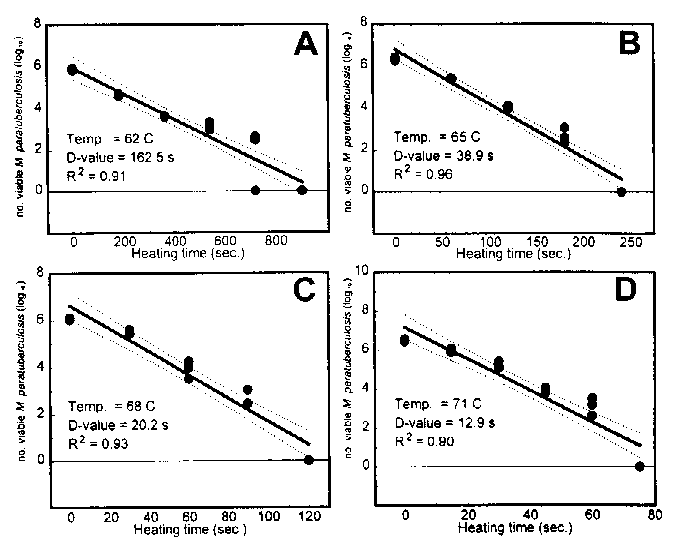
FIG. 4. Thermal death curves for M. paratuberculosis Dominic in milk. (A) 62oC. (B) 65oC. (C) 68oC. (D) 71oC. Data points are log10 counts of M. paratuberculosis cells. Thick solid line, linear regression line; dotted lines, 95% confidence intervals.
FIG. 5. Estimated pasteurization lines for clinical strains of M. paratuberculosis, when the initial concentration was 106 organisms/ml, and for comparable numbers of M. bovis and C. burnetti based on the reports of William et al.34 and Enright et al.13.
| Strain | D-value (s) at: |
Z-value (oC) |
|||
| 62oC | 65oC | 68oC | 71oC | ||
| ATCC 19698 | 324.6a | 55.7a | 23.8a | 13.0 | 6.57 |
| BO45 | 170.3 | 52.6 | 20.9 | 11.9 | 7.75 |
| Dominic | 139.3 | 45.5 | 21.3 | 13.1 | 8.80 |
| Ben | 201.5 | 41.7 | 16.8 | 9.9 | 6.94 |
| Pooled | 179.2 | 44.2 | 19.4 | 12.2 | 7.77 |
aSignificantly different from D-values for other individual strains within temperature column.
| Strain | D-value (s) at: |
Z-value (oC) |
|||
| 62oC | 65oC | 68oC | 71oC | ||
| ATCC 19698 | 119.9a | 70.6a | 22.8 | 16.5a | 9.76 |
| BO45 | 308.9a | 47.7 | 21.7 | 11.7 | 6.51 |
| Dominic | 162.5 | 38.9 | 20.2 | 12.3 | 8.24 |
| Ben | 209.9 | 40.9 | 14.6 | 9.8 | 6.94 |
| Pooled | 228.8 | 47.8 | 21.8 | 11.6 | 7.11 |
aSignificantly different from D-values for other individual strains within temperature column.
|
Initial concentration (organisms/ml) |
Estimated 100% killing time (s) in: | |||||||
| Milk | Lactate solution | |||||||
| 62oC | 65oC | 68oC | 71oC | 62oC | 65oC | 68oC | 71oC | |
| 106 | 1,373 | 287 | 131 | 70 | 1,075 | 265 | 116 | 73 |
| 105 | 1,144 | 239 | 109 | 59 | 896 | 221 | 97 | 62 |
| 104 | 915 | 191 | 87 | 47 | 717 | 177 | 77 | 49 |
| 103 | 686 | 143 | 66 | 35 | 538 | 133 | 58 | 37 |
| 102 | 458 | 96 | 44 | 23 | 358 | 88 | 39 | 25 |
| 101 | 229 | 48 | 22 | 11 | 179 | 44 | 19 | 12 |
| 1 | Bradshaw, J. G., J. T. Peeler, J. J. Corwin, J. M. Tierney, E. P. Larkin, and R. M. Twedt. 1985. Thermal resistance of L. monocytogenes in milk. J. Food Prot. 48:743-745. |
| 2 | Chatterjee, S., and B. Price. 1991. Regression analysis by example, 2nd ed., p. 106-107. John Wiley and Sons, Inc., New York, N.Y. |
| 3 | Chiodini, R. J., and C. A. Rossiter. 1996. Paratuberculosis: a potential zoonosis? Vet. Clin. N. Am. Food Anim. Pract. 12:457-467. |
| 4 | Chiodini, R. J., and J. Hermon-Taylor. 1993. The thermal resistance of Mycobacteriom paratuberculosis in raw milk under conditions simulating pasteurization. J. Vet. Diagn. Investig. 5:629-631. |
| 5 | Chiodini, R. J., H. J. Van Kruinigen, R. S. Merkal, W. R. Thayer, and J. A. Coutu. 1984. Characteristics of an unclassified Mycobacteriom species isolated from patients with Crohn’s disease. J. Clin. Microbiol. 24:966-971. |
| 6 | Chiodini, R. J., H. J. Van Kruinigen, W. R. Thayer, and J. A. Coutu. 1986. Spheroplastic phase of mycobacteria isolated from patients with Crohn’s disease. J. Clin. Microbiol. 24:357-363. |
| 7 | Chiodini, R. J., H. J. Van Kruinigen, W. R. Thayer, R. S. Merkal, and J. A. Coutu. 1984. Possible role of mycobacteria disease in inflammatory bowel disease. Dig. Dis. Sci. 29:1073-1079. |
| 8 | Clark, S. L. 1995. Development and characterization of an avian model of disseminated Mycobacterium avium infection. M.S. thesis. University of Wisconsin-Madison, Madison, Wis. |
| 9 | Dell’Isola, B., C. Poyart, 0. Goulet, J. F. Mougenot, E. Sadoun-Journo, N. Brousse, J. Schmitz, C. Ricour, and P. Berche. 1994. Detection of Mycobacterium paratuberculosis by polymerase chain reaction in children with Crohn’s disease. J. Infect. Dis. 169:449-451. |
| 10 | Donnelly, C. W., and E. H. Briggs. 1986. Psychrotrophic growth and thermal inactivation of L. monocytogenes as a function of milk composition. J. Food Prot. 49:994-998. |
| 11 | Donnelly, C. W., E. H. Briggs, and L. S. Donnelly. 1987. Comparison of heat resistance of Listeria monocytogenes in milk as determined by two methods. J. Food Prot. 50:14-17. |
| 12 | Draper, N. R., and H. Smith. 1981. Applied regression analysis, 2nd ed., p. 241-257.John Wiley and Sons, Inc., New York, N.Y. |
| 13 | Enright, J. B., W. W. Sadler, and R. C. Robert. 1957. Thermal inactivation of Coxiella bumetii and its relation to pasteurization of milk. Public health monograph no.47. U.S. Public Health Service publication no.517. U.S. Government Printing Office, Washington, D.C. |
| 14 | Gitnick, G., J. Collins, B. Beaman, D. Brooks, M. Arthur, T. Imaeda, and M. Palieschesky. 1989. Preliminary report on isolation of mycobacteria from patients with Crohn’s disease. Dig. Dis. Sci. 34:925-932. |
| 15 | Grant, I. R., H. J. Ball, S. D. Neill, and M. T. Rowe. 1996. Inactivation of Mycobacterium paratubercolosis in cows’ milk at pasteurization temperatures. Appl. Environ. Microbiol. 62:631-636. |
| 16 | Lambrecht, R. S., J. Carriere, and M. T. Collins. 1988. A model for analyzing growth kinetics of a slowly growing Mycobacterium sp. Appl. Environ. Microbiol. 54:910-916. |
| 17 | Lisby, G., J. Anderson, K. Engbaek, and V. Binder. 1994. Mycobacterium paratuberculosis in intestinal tissue from patients with Crohn’s disease demonstrated by a nested primer polymerase chain reaction. Scand. J. Gastro-enterol. 29:923-929. |
| 18 | Mcfadden, J. J., J. Collins, B. Baeman, M. Arthur, and G. Gitnick. 1992. Mycobacteria in Crohn’s disease: DNA probes identify the Wood Pigeon strain of Mycobacterium avium and Mycobacterium paratuberculosis from human tissue. J. Clin. Microbiol. 30:3070-3073. |
| 19 | Mcfadden, J. J., P. D. Butcher, R. Chiodini, and J. Hermon.Taylor. 1987. Crohn’s disease-isolated mycobacteria are identical to Mycobacterium para-tuberculosis as determined by DNA probes that distinguish between myco-bacterial species. J. Clin. Microbiol. 25:796-801. |
| 20 | Merkal, R. S., and D. L. Whipple. 1980. Heat inactivation of Mycobacierium bovis in meat products. Appl. Environ. Microbiol. 40:282-284. |
| 21 | Merkal, R. S., K. K. Kopecky, A. B. Larsen, and T. R. Thurston. 1964. Improvements in the techniques for primary cultivation of Mycobacierium paratuberculosis. Am. J. Vet. Res. 25:1290-1293. |
| 22 | Millar, D., J. Ford, J. Senderson, S. Withey, M. Tizard, T. Doran, and J. Hermon.Taylor. 1996. IS900 PCR to detect Mycobacierium paratuberculosis in retail supplies of whole pasteurized cows’ milk in England and Wales. Appi. Environ. Microbiol. 62:3446-3452. |
| 23 | Mishna, D., P. Katsel, S. T. Brown, E. C. A. M. Gilberts, and R. J. Greenstein. 1996. On the etiology of Crohn’s disease. Proc. Natl. Acad. Sci. USA 93:9816-9820. |
| 24 | Moss, T., E. P. Green, M. L. Tizard, Z. P. Malik, and J. Hermon-Taylor. 1991. Specific detection of Mycobacterium paratuberculosis by DNA hybridization with a fragment of the insertional element 1S900. Gut 32:395-398. |
| 25 | Murray, A., J. Oliaro, M. M. T. Schlup, and V. S. Chadwick. 1995. Mycobacterium paratuberculosis and inflammatory bowel disease: frequency distribution in serial colonoscopic biopsies using polymerase chain reaction. Microbios 83:217-228. |
| 26 | Pavias, M. 1990. Thermoresistance of mycobacteria. Acta Vet. Brno 59:65-71. |
| 27 | Sanderson, J. D., M. T. Moss, M. L. V. Tizard, et al. 1992. Mycobacterium paratuberculosis DNA in Crohn’s disease tissue. Gut 33:890-896. |
| 28 | Schroederus, S.S. 1994. Development of an in-vitro method for determining antimicrobial susceptibility of Mycobacterium avium complex. M.S. thesis. University of Wisconsin-Milwaukee, Milwaukee, Wis. |
| 29 | Streeter, R. N., G. F. Hoffsis, S. Bech-Nielson, W. P. Shulaw, and D. M. Rings. 1995. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am. J. Vet. Res. 56:1322-1324. |
| 30 | Stumbo, C. R. (ed.). 1973. Thermobacteriology in food processing, 2nd ed., p.93-120. Academic Press, New York, N.Y. |
| 31 | Sweeney, R. W., R. H. Whitlock, and A. E. Rosenberger. 1992. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 30:166-171. |
| 32 | Taylor, T. K., C. R. Wuks, and D. S. McQueen. 1981. Isolation of Mycobacterium paratuberculosis from the milk of a cow with Johne’s disease. Vet. Rec. 109:532-533. |
| 33 | Thompson, D. E. 1994. The role of mycobacteria in Crohn’s disease. J. Med. Microbiol. 41:74-94. |
| 34 | William, T. H., H. V. Hagstad, and E. Spangler. 1991. Food processing technology, p.45-49. In W. T. Hubbert and H. V. Hagstad (ed.), Food safety and quality assurance. Iowa State University Press, Ames, Iowa. |
*Corresponding author. Mailing address: 2015 Linden Dr. W., Madison, WI 53706. Phone: (608) 263-6920. Fax: (608) 265-6463.