This site is no longer maintained and is presented for archive purposes only
The immune system of the intestinal tract has been extensively studied. The lamina propria of adults is heavy populated with a variety of lymphoid cells: B- and T-lymphocytes, plasma cells, macrophages, mast cells, eosinophils and basophils. In normal subjects the majority of the B-cells are committed to IgA synthesis (90%), the residual 10% being represented (in order of decreasing frequency) by IgM, IgG, IgE and IgD producing plasma cells. These cells, which are located diffusely in the mucosa and more organized in Peyer's patches, together with an intact mucosal layer provide an effective barrier to the antigenic food load, that passes the bowel daily. Small amounts of antigen can transmigrate through the mucosa either by endo/pinocytic processes or via the tight junction complex. The production of antibodies to antigens penetrating the barrier is a physiological process that usually results in systemic tolerance. Changes in these immunological processes, caused by an altered mucosal barrier, an abnormal antigen-antibody or antigen-antigen presenting cell response, may play a role in the pathogenesis of Crohn's disease.
Although many immunological changes have been documented in Crohn's disease, it still is not clear whether these changes are a primary disorder of the immune system or secondary to the inflammatory process; in both cases the initiating process or agent that leads to inflammation remains unknown. In this chapter we will discuss some immunological changes that can be observed in Crohn's disease patients. We will speculate on the pathogenic role of mycobacterial antigens and suggest a novel immuno therapeutical intervention strategy.
In Crohn's disease an increased permeability of the intestinal mucosa for larger molecules may exist1,2, probably due to an abnormal tight junction structure3. Various (food derived-?) antigens can pass the mucosal layer by pino- or endocytosis, but oral and systemic tolerance for most of them is derived early in life by respectively mucosal IgA production and specific non-responsiveness of the systemic immune system to luminal antigens. It is not clear whether the permeability for very large molecular structures such as mycobacteria or mycobacterial antigens is increased in Crohn's disease. Besides a primary change in permeability it is also possible that penetration of antigens in the inflamed mucosa is a secondary process which perpetuates a destructive primary immune response. The supposed primary increased permeability is not obligate for immune activation, because enterocytes may exhibit antigen presenting capacities, shown by there expression of HLA-DR4,5,6.
The importance of T-lymphocytes within the normal gut and gut-associated lymphoid tissue is generally accepted: their precise role, however, is uncertain. In the lamina propria most T-lymphocytes express the CD4 (helper) phenotype, whereas in the epithelial layer the CD8 (cytotoxic/suppressor) subtype dominates. The CD4 + cells are predominantly of the helper-inducer and cytolytic subtype, and less frequently of the suppressor-inducer phenotypes7. In Crohn's disease there is an influx of inflammatory cells into the diseased area and local production of soluble mediators of inflammation has been shown8,9. The pivotal role of T-cells in Crohn's disease is well known10. There is an increased number of mucosal T-cells, but the CD4 to CD8 ratio is the same as in control subjects11. Earlier immuno-histopathological reports could not show an increase in T-cell activation markers12,13. This in contrast to the fact that mononuclear cells isolated from mucosa from Crohn's disease patients did show a slight increase in the expression of these markers14. Later studies showed that activated T-cells are abundantly present the submucosa of children with Crohn's disease, in contrast to mucosa of ulcerative colitis patients15,16. T-cell activation is not only evident in histological specimens17,18 but can also be shown in peripheral blood: even the serum level of soluble inter-leukin-2 receptor is increased 19,20 The total numbers of CD4 and CD8 positive lymphocytes in peripheral blood of Crohn's disease to patients are normal, but the T-cells are activated as they express CD25 (interleukin-2 receptor) on their surface21.
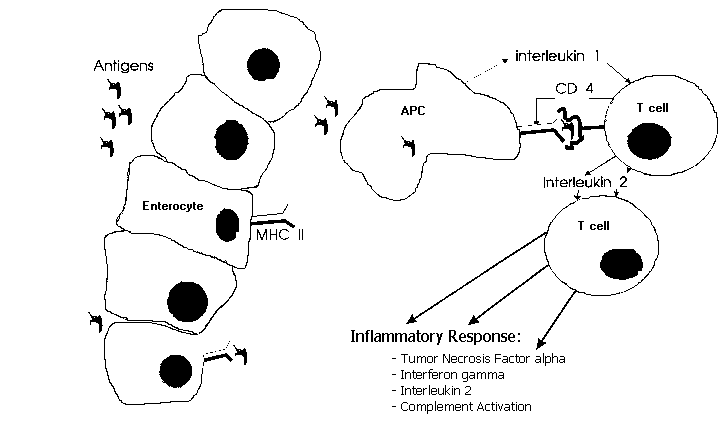
In case of HLA class II restricted antigen presentation, as in type IV immune response, an antigen (fig. 1) is processed by the antigen presenting cell (enterocyte, mononuclear cell, dendritic cell) and presented on its surface by a MHC class II molecule. This complex is recognized by the T helper 1 (Th 1) cell receptor. Th 1 cells mediate delayed type hypersensitivity22. Additional bindings (integrins) and a costimulatory signal (interleukin- 1) are necessary for T-cell activation23. The antigen(s) to which those T-cells react are still unknown. Their identification however would be a major step forward in our understanding of Crohn's disease. Mycobacteria elicit a cellular immune response in their hosts. This response usually leads to protective immunity, but may sometimes be accompanied by immunopathology due to delayed type hypersensitivity (like in tuberculoid leprosy). Mycobacterial antigens are capable of creating a granulomatous inflammation24, in which mononuclear cells play an important role. Living mycobacteria are not necessary in this process. Mycobacterial antigens can be shown in mucosa of Crohn's disease patients25 and Thayer showed that serum antibodies to mycobacterial antigens are more often present in Crohn's disease patients26. Mycobacteria selectively induce human T-cells with a Th 1-like cytokine profile27. Lymphocytes from Crohn's disease patients show marked suppressor activity when stimulated with M. paratuberculosis28. These immunological findings may explain why Crohn's disease is a transmural inflammation: the mycobacterial antigen is passing through the mucosa one way or another and subsequently processed by the antigen presenting cell (APC). Then in the lamina propria the T-cell receptor of the T-helper (CD4 +) cell recognizes the antigen-MHC II complex. This results in T-cell activation and a granulomatous transmural inflammation moderated by tumor necrosis factor alpha, t interferon, and interleukin-2. Its however not clear what causes the perpetuating immune response and subsequent inflammation.
The ideal immunosuppressive therapy would be either to neutralize the causative antigen, or to delete all reactive T-cell clones, without affecting the normal immune response. Somewhat less specifically, one could interfere with the antigen presenting process. As can be seen in figure 1. intervening in the antigen presenting pathway provides opportunities for immunotherapy. Because the antigen which causes the immune response in Crohn's disease is still unknown, no vaccination, antibodies or desensibilisation are available.
As T-cells play a central role in all immune responses, they are a primary target for any immuno-therapeutic intervention strategy. For many years polyclonal antibodies that suppress T-cell functions have been investigated as immunosuppressive agents in a variety of diseases. With the development of monoclonal antibodies, specific immunotherapy directed against T-cells became available and had promising results in both animal models and (auto-)immune diseases29,30. Monoclonal antibody treatment has largely been applied clinically in cancer and modulation of the immune response to produce immunosuppression for treatment of autoimmune and graft versus host diseases. It is also very effective in the treatment of acute allograft rejection31. Human monoclonal antibodies have been used in both viral and bacterial infections32. Some monoclonal antibodies are now registered for clinical use, for example OKT3 in allograft rejection. Problems with monoclonal antibody therapy were recognized soon because anti-(murine-) antibody production limited therapeutic possibilities33. Furthermore there are many side effects in murine- and non specific (for example OKT3-) monoclonal antibody therapy. New "humanized" chimeric and recombinant monoclonal antibodies have promising therapeutic prospects. Interference with the antigen presentation by blocking the T-cell receptor, the CD4 receptor, interleukin-1B pathway or the interleukin-2 receptor all are therapeutical options. Other , less specific, strategies consist of blockade of the inflammatory response by antibodies directed against tumor necrosis factor-alpha, interleukin-2, interferon-t or their receptors. Currently some of these monoclonal antibody therapies are tested in clinical trials.
The treatment of Crohn's disease with Corticosteroids, Azathioprine/6-mercaptopurine34, and 5-Aminosalicylic acid is well accepted and needs no further discussion in this chapter.
Cyclosporine is a novel immunosuppressant, widely used in organ transplantation, that has a selective effect on T-cell media immune responses35. Cyclosporine is able to reduce expression of Class II molecules on the epithelium in a dramatic way. It also reduces IL-2 and hence t-interferon production. Cyclosporine has proven to have a beneficial effect in active chronic Crohn's disease patients who were resistant to or intolerant of corticosteroids36. Cyclosporine therapy during three month was well tolerated and no serious adverse events were observed, but is associated with a risk of nephropathy.
Anti-CD4 treatment in Crohn's disease seems rational in review of the antigen presenting pathway and T-cell activation in Crohn’s disease, as described above. Depletion of CD4 + cells is correlated with rapid improvement in a prototypic model of T-cell-mediated autoimmunity: experimental allergic encephalomyelitis37. Reports of remissions of Crohn's disease in patients infected with the HIV virus provide additional arguments for anti-CD4 treatment in Crohn’s disease38. In these cases remission of Crohn's disease occurred simultaneously with a decrease in CD4 cells. In phase I trials, rheumatoid arthritis, which is also characterized by a granulomatous inflammation and T-cell activation, responds to the treatment with murine monoclonal antibodies against the CD4+ (T helper) cells39,40. The effect on remission of symptoms after one week of therapy lasted 3 weeks to more than 5 months. Only short-lasting low grade fever on the first day of treatment was seen. However in six out of eight patients an anti-mouse-Ig response developed. This can cause problems (ineffectiveness, allergic response) in longterm treatment of these chronic diseased patients. Currently we are evaluating a chimeric (mouse/human) anti-CD4 monoclonal antibody in Crohn's disease patients, with promising results and without serious side effects. These antibodies have a human IgG constant part and a mouse anti-CD4 variable part. We assume that humanizing the antibody causes less antigenetic response and a longer half live.
Both mycobacterial and Crohn's disease are characterized by a granulomatous, transmural inflammation of the bowel. Tumor necrosis factor alpha (TNF-alpha) plays a very important and perhaps pivotal role in granulomatous diseases41,42,43. Blocking TNF-alpha prevents the formation of granuloma's44. TNF-alpha is in increased amounts present in mucosa and stool of patients with active IBD45,46. TNF-alpha can be controlled by reducing its production, TNF-neutralizing antibodies or by blocking the TNF-receptor. Several studies, using murine or murine/human chimeric antibodies, have been initiated in septicemia, and the results from a phase I study have been published47. To our knowledge, anti-TNF therapy has not been investigated in Crohn's disease.
Pentoxifylline (a xanthine derivative) has been described to counteract endotoxin induced effects in different animal models48,49. Pentoxifylline inhibits in macrophage cultures the synthesis of mRNA for TNF-alpha50 and therefore is an alternative for anti-TNF antibody therapy. Pentoxifylline is also able to inhibit the inflammatory action of interleukin-1 and TNF-alpha on neutrophil function51. No data are available on the effect of pentoxifylline in granulomatous diseases. Thalidomide, which is presently used to treat some forms of leprosy, has also been shown to specifically inhibit transcription of the TNF gene in monocytes52.
As shown in fig. 1 interleukin-l (IL-1) and interleukin-2 (IL-2) play a role in HLA class II restricted antigen presentation and (auto-)immune response. This is confirmed in IBD by studies showing enhanced IL-2 and IL-lB production53 in mononuclear cells isolated from the mucosa and biopsy specimens17. In autoimmune diseases and in Crohn's disease serum soluble IL-2 receptor concentration is raised, indicating T-cell19. Intestinal mucosal mononuclear cells of Crohn's disease patients produce only one-third of the amount of interleukin-2 generated by control cells after stimulation, but were able to exhibit comparable cytotoxicity54. This is probably due to either an increased number of interleukin-2 responsive cells or an exaggerated reactivity to interleukin-2. Lack of IL-2 production seems to play a role in the disability of a granulomatous response to Schistosoma mansoni in mice55. Recombinant IL-2 therapy reversed this diminished response. Its not clear what the exact function of the granuloma formation in Crohn's disease is; Is it an exaggerated immune response to antigens or a decreased response to bacteria? Counteracting IL-2 production or neutralizing its effects by monoclonal antibodies directed against its receptor, may be an option for immunotherapy of Crohn's disease especially when we consider the antigen c.q. abnormal immune response. Monoclonal antibodies against IL-2 receptor are used in the prophylactic treatment of human kidney allograft recipients, with good result and only few side effects56. No studies are as yet available monoclonal antibody treatment against the IL-1 receptor.
| 1 | Katz KD, Hollander D, Vadheim CM, et al. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology 1989;97 :927-31. |
| 2 | Howden CW, Robertson C, Ducan A, Morris AJ, Russel RI. Comparison of different measurements of intestinal permeability inflammatory bowel disease. Am J Gastroenterol 1991 ;86: 1445-9. |
| 3 | Madara JL, Moore R, Carison S. Alteration of intestinal tight junction structure and permeability by cytoskeletal contraction. Am Phy Soc 1987;C854-61. |
| 4 | Ouyang Q, EI-Youssef M, Yen-Lieberman B, Sapatnekar W, Youngman KR, Kusugami K, et al. Expression of HLA-DR antigens in inflammatory bowel disease mucosa: Role of intestinal lamina propria mononuclear cell-derived interferon-t. Dig Dis Sci 1988;33: 1528-36. |
| 5 | Cuvelier C, Mielants H, Vos de M, Veys E, Roels H. Major histocompatibility complex class II antigen (HLA-DR) expression by ileal epithelial cells in patients with seronegative spondylarthropathy Gut 1990;31:545-9. |
| 6 | Mayer L, Eisenhardt D, Salomon P, Bauer W, Plous R, Piccinini L. Expression of class II molecules on intestinal epithelial cells in humans. Differences between normal and inflammatory bowel diseases. Gastroenterology 1991; 100:3-12. |
| 7 | James SP, Fiocchi C, Greaff AS, Strober W. Phenotypic analysis of lamina propria lymphocytes. Predominance of helper-inducer and cytolytic T-cell phenotypes and deficiency of suppressor-induced phenotypes in Crohn's disease and control patients. Gastroenterology 1986;9 1:1483-9. |
| 8 | Rappaport H, Burgoyne FH, Smetana HF. The pathology of regional enteritis. Milit Surg 1951; 109:463-502 |
| 9 | Zifronini A, Treves AJ, Sachar DB, Rachmilewitz D. Prostanoid synthesis by cultured intestinal epithelial and mononuclear cells in inflammatory bowel disease. Gut 1983;24:659-65. |
| 10 | Ferguson A. Why study T cell subsets in Crohn's disease? Gut 1983;24:687-91. |
| 11 | Selby WS, Janossy G, Bofill M, Jewell DP. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut 1984;25 :32-40. |
| 12 | Konttinen YT, Bergroth V, Nordstrom D, Segerberg-Konttinen M, Seppala K, Salaspuro M. Lymphocyte activation in vivo in the intestinal mucosa of patients with Crohn's disease. J Clin Lab Immunol 1987;22:59-63. |
| 13 | Fais S, Pallone F, Squarcia O, et al. HLA-DR antigens on colonic epithelial cells in inflammatory bowel disease: 1. Relation to the state of activation of lamina propria lymphocytes and to the epithelial expression of other surface markers. Clin Exp Immunol 1987;68:605-12. |
| 14 | Pallone F, Fais S, Squarcia 0, Biancone L, Pozzilli P, Boivirant M. Activation of peripheral blood and intestinal lamina propria lymphocytes in Crohn's disease. In vivo state of activation and in vitro response to stimulation as defined by expression of early activation antigens. Gut 1987;28:745-53. |
| 15 | Choy MY, Walker-Smith JA, Williams CB, MacDonald TT. Differential expression of CD25 (interleukin-2 receptor) on lamina propria T cells and macrophages in the intestinal lesions in Crohn's disease and ulcerative colitis. Gut 1990;31:l365-70. |
| 16 | Monk TJ, Spencer J, Cerf-Bensussan N, MacDonald TT. Activation of mucosal T-cells in situ with anti-CD3 antibody: phenotype of activated T-cells and their distribution within the mucosal micro-environment. Clin Exp Immunol 1988;74:216-22. |
| 17 | Brynskov J, Tvede N, Andersen CB, Vilien M. Increased concentrations of interleukin 1B, interleukin-2, and soluble interleukin-2 receptors in endoscopic mucosal biopsy specimens with active inflammatory bowel disease. Gut 1992;33 :55-58. |
| 18 | Meuler CH, Knofiach P, Zielinski CC. T-cell activation in Crohn's disease. Gastroenterology 1 990;98 :639-46. |
| 19 | Crabtree JE, Juby LD, Heatley RV, Lobo AJ, Bullimore DW, Axon ATR. Soluble interleukin-2 receptor in Crohn's disease: relation of serum concentrations to disease activity. Gut 1990;3 1:1033-6. |
| 20 | Mahida YR, Callager A, Kurlak L, Hawkey CY. Plasma and tissue interleukin-2 receptor level in IBD. Clin Exp Immunol 1990;82:75-80. |
| 21 | Senju M, Hulstaert F, Lowder J, Jewell DP. Flow cytometric analysis of peripheral blood lymphocytes in ulcerative colitis and Crohn's disease. Gut 1991;32:779-83. |
| 22 | Cher DJ, Mosman TR. Two types of murine T cell clone. II. Delayed type hypersensitivity is mediated by Th1 clones. J Immunol 1987; 138:3688-94. |
| 23 | Ashwell DJ, Fox BS, Schwartz RH. T cell recognition of antigen and Ia molecules involves a trimolecular complex. in Processing and presentation of antigens 1988;Academic Press, Inc London |
| 24 | Stewart-Tull DES. Immunologically important constituents of mycobacteria: adjuvants. in Biology of Mycobacteria Vol 2.1983 Academic Press London. IBSN 0-12-582302-9 |
| 25 | Blaauwgeers JL, Das PK, Slob AW, Houthoff HJ. Human gut wall reactivity to monoclonal antibodies against M. avium glycolipid relation to Crohn's disease (preliminary results). Acta Leprol (Genev 1989;7 Suppl 1:138-40. |
| 26 | Thayer WR Jr, Coutu JA, Chiodini RJ, Van Kruiningen HJ, Merkal RS. Possible role of mycobacteria in inflammatory bowel disease. I Mycobacterial antibodies in Crohn's disease. Dig Dis S 1984;29: 1080-5. |
| 27 | Haanen JBAG, Waal de Malefijt R, Res PCM, Kraakman EM, Ottenhof THM, Vries RRP, et al. Selection of a human T helper type1-like T cell subset by mycobacteria. J Exp Med 1991;174:583-92. |
| 28 | Ebert EC, Bhatt BD, Lui S, Das KM. Induction of suppressor cells by Mycobacterium paratuberculosis antigen in inflammatory bowel disease. Cli Exp Immunol 1991;83:320-5. |
| 29 | Atalla L, Linker-Israeli M, Steinman L, Rao NA. Inhibition of autoimmune uveitis by Anti-CD4 antibody. Invest Ophthalmol Vis Sci 1990;3 1:1264-70. |
| 30 | Hafler DA, Faltis RJ, Dawson DM, Schlossman SF, Reinherz EL, Weiner HL. Immunologic responses of progressive multiple sclerosis( patients treated with an anti-T-cell monoclonal antibody, anti-T12 Neurol 1986;36:777-84. |
| 31 | Ortho Multicenter Transplantation Group. N Eng J Med 1985;313:33742. |
| 32 | Ziegler EJ,. N Eng J Med 1991;324:429-. |
| 33 | Catane R, Longo DL. Isr J Med Sci 1988;24:471-. |
| 34 | O'Brien JJ, Bayless TM, Bayless JA. Use of Azathioprine or 6-Mercaptopurine in treatment of Crohn's disease. Gastroenterology 1991; 101:39-46. |
| 35 | Hess AD, Colombani PM. Cyclosporin: mechanism of action: in vitro studies. Prog Allergy 1986;38:198-221. |
| 36 | Brynskov J, Freund L, Rasmussen SN, Lauritsen K, Schaffalitzky de Muckadell 0, Williams N, et al. A placebo-controlled, double-blind, randomized trial of cyclosporine therapy in active chronic Crohn’s disease. N Engl J Med 1989;321:845-50 |
| 37 | Alters SE, Sakai K, Steinman L, Oi VT. Mechanisms of anti-CD-mediated depletion and immunotherapy. A study using a set of chimeric anti-CD4 antibodies. J Immunol 1990;144:4587-92. |
| 38 | James SP. Remission of Crohn's disease after human immunodeficiency virus infection. Gastroenterology 1 988;95:1667-9. |
| 39 | Herzog C, Walker C, Müller W, Rieber P, Reiter C,Rietmüller G, al. Anti-CD4 antibody treatment of patients with Rheumatoid arthritis. I. Effect on clinical course and circulating T Cells. J Autoimmunity 1989;2 :627-42 |
| 40 | Walker C, Herzog C, Rieber P, Rietmu~ller G, Mu~ller W, Pichler W Anti-CD4 antibody treatment of patients with Rheumatoid arthritis: II. Effect of in vivo treatment on in vitro proliferative response of CD4 cells. J Autoimmun 1989;2:643-9. |
| 41 | Asano M, Minagawa T, Ohamichi M, Hiraga Y. Detection of endogenous cytokines in sera or lymph nodes obtained from patients with sarcoidosis. Clin Exp Immunol 1991:84:92-6. |
| 42 | Ruco LP; Stoppacciaro A; Pomponi D; Boraschi D; Santoni A; Tagliabue A; Uccini S; Baroni CD. Immunoreactivity for IL-1 beta and TNF alpha in human lymphoid and nonlymphoid tissues. Am J Pathol 1989;135:889-97. |
| 43 | Dunn CJ, Hardee MM, Staite ND. Acute and chronic inflammatory responses to local administration of recombinant IL-i alpha, IL-beta, TNF alpha, IL-2 and Ifn gamma in mice. Agents Actions 1989;27:290-3. |
| 44 | Kindler V, Sappino AP, Grau G, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 1989;56:731-40. |
| 45 | MacDonald TT, Hutchings P, Choy M-Y, Murch S, Cooke A. Tumor necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Cli Exp Immunol 1990;81:301-5. |
| 46 | Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 1 992;339:89-91 |
| 47 | Exley AR, Cohen J, Buurman W, et al. Monoclonal antibody to TNF in severe septic shock. Lancet 1990;335: 1275-7 |
| 48 | Schönharting MM, Schade UF. The effect of pentoxyfilline in septic shock - new pharmacological aspects of an established drug. J Med 1989;20:97-105. |
| 49 | Schade UF. Pentoxyfylline increases survival in murine endotoxine shock and decreases formation of tumor necrosis factor.Circ Shock 1990;31:171-81. |
| 50 | Stricter RM, Remick DG, Ward PA et al. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxyfilline. Biochem Biophys Res Comm 1988;155:1230-6. |
| 51 | Sullivan GW, Carper HT, Novick WJ, Mandell GL. Inhibition of inflammatory action of interleukin-1 and tumor necrosis factor (alpha) on neutrophil function by Pentoxifyll inc. Infect Immun 1988;56: 1722-9. |
| 52 | Sampaio EP, Sarno EN, Galilly R, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med 1991;173:699-703. |
| 53 | Mahida YR, Wu K, Jewel DP. Enhanced production of interleukin 1-B by mononuclear cells isolated from mucosa with active ulcerative colitis or Crohn's disease. Gut 1989;30:835-8. |
| 54 | Kusugami K, Youngman KR, West GA, Fiocchi C. Intestinal immune reactivity to interleukin 2 differs among Crohn's disease, ulcerative colitis. and controls. Gastroenterology 1989;97: 1-9. |
| 55 | Mathew RC, Ragheb S, Boros DL. Recombinant IL-2 therapy reverses diminished granulomatous responsiveness in anti-L3T4-treated, Schistosoma mansoni-infected mice. J Immunol 1990; 144:4356-61. |
| 56 | Peyronnet P, Le Mauff B, Hourmant M, Cantarovich D, Dudigeon P, Olive D, et al. Prophylactic treatment of human kidney allograft recipients with a monoclonal antibody (33B3. 1) directed against interleukin 2 receptor. Transplantation Procedings 1 988;20: 300-2. |

Figure 1:The antigens on the luminal side of the mucosa transmigrate through the epithelial layer, either by endo-/pinocytosis or by the tight junction complex. In case of HLA-restricted antigen presentation as in Crohn's disease the antigen is processed by the antigen presenting cell (APC) and presented on its surface by the class II major histocompatibility complex (MHC II). The antigen presenting cell can either be an enterocyte, monocyte/macrophage or dendritic cell. The MHC II-antigen complex is recognized by the T-cell receptor. Additional binding by the CD4 molecule (integrin) and a costimulatory signal are necessary for T-cell activation. This in turn leads to the inflammatory response.