This site is no longer maintained and is presented for archive purposes only
The combined effect of clarithromycin and rifabutin against Mycobacterium avium multiplying either within human monocyte-derived macrophages or extracellularly in a liquid medium was additive: both MICs and MBCs were twofold lower for the combination than they were for each drug alone. Prolonged exposure for 4 weeks of M. avium-infected macrophages to combined concentrations that were only twofold greater than the MICs resulted in a 100-fold decrease in the number of viable bacteria, while in the drug-free controls a 100-fold or greater increase in comparison with the initial viable counts took place. Comparison of this effect with the results of the prolonged exposure to each drug alone suggested that under these experimental conditions rifabutin enhanced the antimicrobial activity of clarithromycin against intracellular bacteria. At the same time, inhibition of intracellular growth by a 2-h pulsed exposure of the infected macrophages to the combination of the two drugs was not different from the effect induced by Clarithromycin alone. In conclusion, clarithromycin played the major role in the antimicrobial activity of the tested combination, while rifabutin may have enhanced this effect during a prolonged exposure of the intracellular bacteria to these two agents.
Combinations of Clarithromycin with a variety of other drugs have been investigated in vitro in cell-free culture media and macrophages 3,7,9,10,16,17. The overall analyses of these data indicated that the interaction in these combinations tested against Mycobacterium avium was additive rather than synergistic 2. The effect of this combination for the treatment and prevention of disseminated M avium infection in patients with AIDS is under investigation. The aim of the present study was to evaluate the activity of Clarithromycin in combination with rifabutin against M avium in a cell-free culture medium and human monocyte-derived macrophages. The study included an evaluation of the antimicrobial activity during prolonged exposure and the effect of a drug combination given to infected macrophages by pulsed exposure.
Antimicrobial agents. Rifabutin (Adria Laboratories, Pharmacia, Columbus, Ohio) was dissolved first in methanol (640 µg/ml) and was then diluted further in distilled water to make appropriate working solutions. Clarithromycin (Abbott Laboratories, Abbott Park, Ill.) was first dissolved in dimethyl sulfoxide (1,280 µg/ml) and was then diluted further in phosphate buffer (pH 6.5).
Test strains. Three strains identified as M avium by the Gen-Probe (San Diego, Calif.) technique were used for the studies. These strains, isolated from blood samples obtained from patients with AIDS, were used in our previous studies 8,12,13. They were preserved in frozen 7H9 broth culture aliquots at -70oC. For each experiment, a subculture in 7H9 broth was made from a frozen aliquot.
Antimicrobial activity against extracellular bacteria. The MIC was defined as the lowest drug concentration that inhibited more than 99% of the bacterial population within a period of 8 days. The working solutions of the drugs alone and in combination were added in a volume of 0.1 ml each per 4.0 ml of 7H12 broth in 12B vials (Becton Dickinson Diagnostic Instrument Systems, Sparks, Md.). The inoculum contained 104 to 105 CFU/ml. The vials were incubated at 37oC for 8 days and samples were taken on days 0, 3, 5, and 8 for plating on 7H11 agar plates to determine the number of CFU per milliliter in drug-containing and drug-free vials. More details were given in our previous publications 6,8. To test the activities of the drug combinations, the combinations containing each drug at one, one-half, one-quarter, and one-eighth MIC were added to respective 12B vials. At the same time the drugs were tested again singly as concomitant controls. All experiments were done in duplicate, and each number of viable bacteria represented a mean of four measurements. The standard error in all experiments did not exceed 5%.
M. avium-infected human macrophages. Monocytes were separated from a by-product of healthy donors' blood used for platelet preparation. This product was provided under the generic name leucopac by the Bonfils Blood Center (Denver, Colo.). The donors who provided blood for the preparation of this product were selected by the conventional standards by blood bank centers, including preliminary testing for human immunodeficiency virus and hepatitis B virus. The mononuclear cells were separated by Ficoll-Hypaque gradient centrifugation at 800 X g for 15 min. The mononuclear cell band was transferred to RPMI 1640 (GIBCO). After centrifugation 250 X g for 10 min at room temperature, the pellet was washed once, resuspended in RPMI 1640, and adjusted to 107 cells per ml. The suspension was placed in 35-mm plastic petri plates (Becton Dickinson Labware, Lincoln Park, N.J.) in two spots approximately 0.05 ml each, resulting in monolayers containing about 2 x 105 cells. The plates were incubated at 37oC for 1 h to allow the cells to adhere and were then washed with RPMI 1640 to remove the nonadherent cells. The cells were incubated in RPMI 1640 containing 5% human nonheated serum (1.5 ml per plate). After incubation for 7 days at 37oC in 7% CO2 the monocytes were considered to have matured into a macrophage monolayer12. The medium was removed from the plates and was replaced with bacterial suspensions containing about 1 x 106 CFU per monolayer (per 2 x 105 macrophages). After incubation for 1 h the plates were washed three times to remove the extracellular bacteria. The infected macrophages were incubated in RPMI 1640 supplemented with 1% human nonheated serum at 37oC in the presence of 7% CO2.
Inhibitory and bactericidal activities against intracellular bacteria. The MIC was defined as the lowest drug concentration present in the culture medium that completely inhibited (>99%) bacterial multiplication in macrophages within a period of 7 days. This period of observation in the macrophage model was selected by us and others 12,16,17 on the basis of the fact that sufficient intracellular multiplication of M avium in the drug-free controls with up to a 100-fold increase in viable counts occurred within 7 days of cultivation. The MBC was defined as the lowest drug concentration that decreased the viable bacterial counts in macrophages more than 100-fold within the same period of cultivation. Various concentrations of the drugs alone or in combination were added to the cultures of infected macrophages. After an appropriate period of incubation the medium from the plates was discarded and the monolayers were lysed by exposure for 10 min to a 0.25% solution of sodium dodecyl sulfate at 1.0 ml per plate. After the suspension was transferred to the tube, the plates were rinsed with 1.0 ml of 7H9 broth containing 20% bovine serum albumin, and the rinse was added to the same tube. The viable counts per monolayer were determined by colony counting on 7H11 agar plates inoculated with serial dilutions of the macrophage lysate at days 0, 4, and 7 12,13. The drug concentrations were selected according to our preliminary experiments with each drug alone. The combinations contained each drug at one, one-half and one-quarter the MIC or MBC. The MIC and MBC of each drug singly were retested at the same time.
Long term exposure of infected macrophages to drugs alone and in combination. The experiments in which infected macrophages were exposed to the drugs alone and in combination were arranged in the same way as those for MIC and MBC determinations, but the period of observation was 4 weeks. The medium containing appropriate drug concentrations was replaced weekly, and the samples for determining the number of viable bacteria were also taken once a week.
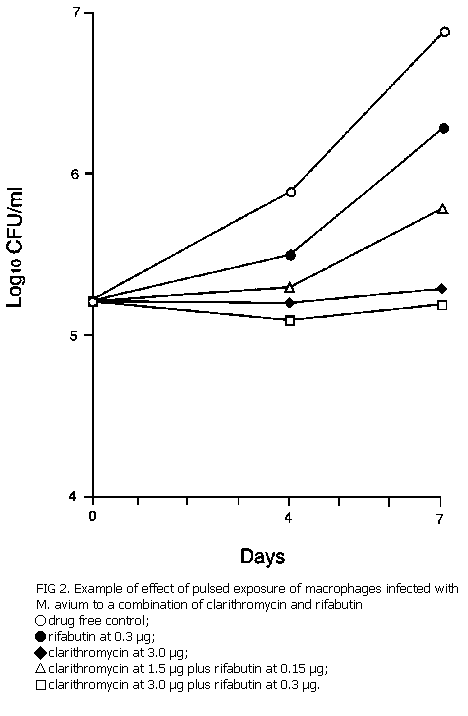
Pulsed exposure of infected macrophages. The peak concentration in blood (Cmax) that can be achieved 2 h after the administration of a dose of 500 mg of clarithromycin is about 4 µg/ml.2 For rifabutin, the Cmax achievable at 2 to 3 h after the administration of 300 mg is 0.4 µg/ml.8 The highest concentrations that can be maintained for a period of 2 h, 1 h before and 1 h after Cmax, were estimated from the pharmacokinetic curves13: 3.0 µg/ml for clarithromycin and 0.3 µg/ml for rifabutin. On the basis of these estimates, the M avium-infected macrophages were exposed for 2 h to a combination of both drugs and to each drug singly at the following concentrations: clarithromycin, 3.0 µg/ml singly and 1.5 µg/ml in the combination; rifabutin, 0.3 µg/ml singly and 0.15 µg/ml in the combination. After the pulsed exposure, the medium was completely removed and was replaced with fresh drug-free medium, and the infected macrophages were cultivated for 7 days13,15.
MICs and MBCs. Experiments in cell-free medium showed that the MICs of Clarithromycin and rifabutin in combination were not more than twofold lower than those of each drug alone (Table 1). This resulted in fractional inhibitory concentrations (FICs) of 1.0 to 2.0, indicating an additive effect at best 5,11. In no single experiment was the FIC 0.5 or less, and therefore, we concluded that no synergistic interaction took place in experiments with extracellular bacteria. The MICs of the drug combination against intracellular bacteria were two-fold lower than the MICs of each drug singly, showing a clear additive effect, with the FIC equal to 1.0 (Table 2). A similar additive effect was found when the MBCs were determined for the intracellular bacteria (Table 3), with MBC/MIC ratios of 64 for Clarithromycin and 8 to 16 for rifabutin.
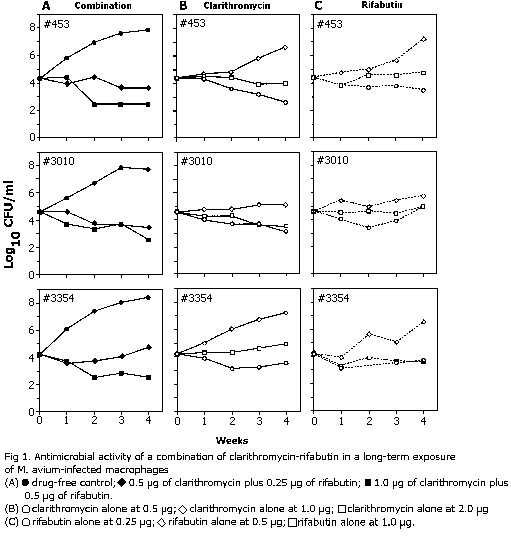
Antimicrobial activity in long-term observations. Ongoing multiplication of bacteria in drug-free macrophages was evident throughout the 4-week period of observation, but it was most striking within the first 3 weeks of cultivation, during which there was more than a 1,000-fold increase in the number of viable bacteria (Fig. 1). A further minimal increase was noted during the fourth week, in which bacterial counts reached 5.4 x 107 to 2.3 x 108 organisms per monolayer. Under these conditions, the MIC of each drug tested alone was the same as those in experiments conducted with only a 1-week period of observation: 1.0 µg/ml for Clarithromycin and 0.5 µg/ml for rifabutin (Table 1). The same effect, and in some instances a decline in the number of bacteria, was achieved when concentrations of one-half the MIC of each drug were used in combination, which reconfirmed the previously noted additive inhibitory effect by the combination of the two drugs, this time observed during a prolonged period. At the same time, an increase in the concentrations of clarithromycin and rifabutin to two times the MICs (2.0 and 1.0 µg/ml, respectively) reduced the number of intracellular bacteria over the 4-week period of exposure in experiments with all three strains (Fig. 1). When the two drugs were combined at concentrations of 1.0 µg of clarithromycin per ml and 0.5 µg of rifabutin per ml, a 100-fold decrease in the number of viable bacteria was detected during 4 weeks of exposure. The concentrations reducing the number of bacteria 100-fold were only twofold greater than those producing just inhibition of growth, a striking difference from the MBC/MIC ratios determined in experiments with a 1-week exposure, as described above. In experiments with strain 453, a prolonged exposure of the infected macrophages to clarithromycin alone in a concentration twofold greater than the MIC also produced some decline in the number of bacteria, similar to that observed in the combination with rifabutin (Fig. 1).
Pulsed exposure of infected macrophages. A single 2-h pulsed exposure to 3.0 µg of clarithromycin per ml alone produced an extended inhibition of growth for a duration of 7 days. Exposure for 2 h to 0.3 µg of rifabutin per ml alone resulted in only partial inhibition of growth, and only during the first 4 days of cultivation. In these experiments, rifabutin barely enhanced the effect of clarithromycin (Fig. 2); combination of clarithromycin at 1.5 µg/ml with rifabutin 0.15 µg/ml was less effective than clarithromycin at 3.0 µg/ml alone, and the addition of 0.3 µg of rifabutin per ml to 3.0 µg/ml of clarithromycin per ml did not enhance the effect of 3.0 µg clarithromycin per ml alone.
The results of the in vitro interaction of clarithromycin with other antimicrobial agents against M. avium are quite contradictory. Some investigators have suggested the possibility of synergistic inhibitory interaction for at least some of the clinical isolates when they are exposed to combinations of clarithromycin with rifabutin, amikacin, or ethambutol10. When clarithromycin was combined with sparfloxacin, a synergistic interaction was reported for only one of seven strains17, and only an additive interaction at best was demonstrated for another 9. A combination with gentamicin was additive7. A combination of clarithromycin with rifampin and ethambutol led to a definite decrease in the MIC compared with those of each drug alone, but it is not very clear whether the interactions of these two drugs with clarithromycin were synergistic or additive 3,16. The only additive effect was reported with a combination of clarithromycin with sparfloxacin and amikacin, but a combination with sparfloxacin and rifampin may have been synergistic for some strains17. Despite the inconsistency of the results obtained in different studies by different techniques, the interaction of clarithromycm with other antimicrobial agents was at least additive2. The combination of Clarithromycin with other agents in the therapy of patients with M. avium infections is especially important for the prevention of the emergence of resistance to clarithromycin. This benefit can be determined in clinical trials only, while the in vitro studies are usually targeted to determining the type of interaction among the agents combined. We concentrated our efforts on clarification of the issue of interaction in only one drug combination, Clarithromycin plus rifabutin. This choice was made because this combination has been selected by several scientific groups for clinical trials currently being conducted in patients with AIDS either for preventive therapy or the treatment of disseminated M. avium infection. Preference for this combination was given because both agents have recently been approved by the U.S. Food and Drug Administration for either prophylaxis (rifabutin) or the treatment (clarithromycin) of M. avium infection.
The present study has shown that the interaction between clarithromycin and rifabutin is not synergistic but is only additive against either extracellular bacteria in a cell-free liquid medium or intracellular bacteria within human macrophages. This interaction led to a twofold reduction in the MICs and MBCs in comparison with the same values for each drug alone. While the MICs of each drug in the combination were less than the peak concentrations attainable in blood, the MBCs were greater than this level. Because of the high MBC and MBC/MIC ratios, neither Clarithromycin nor rifabutin should be considered bactericidal agents against M. avium, even in combination. These data have confirmed the results of our previous studies on the activity of each drug alone 6,8.
There have been speculations that the accumulation of drugs in macrophages may change this balance to result in bactericidal activity against the bacteria multiplying intracellularly 2,10. We confirmed previously that Clarithromycin concentrations in macrophages can be 15- to 17-fold greater than those in the extracellular fluid 14. In spite of this, the concentrations in the extracellular fluid that were required to inhibit the intracellular bacterial growth were in the same range as those that were active against the extracellular bacteria. We speculate that despite accumulation within macrophages Clarithromycin may not have greater antimycobacterial activity as a consequence of its relative inactivity in the acidic milieu within phagolysosomes. The MICs of various rifamycins, including rifabutin, against intracellular mycobacteria (Mycobacterium microti) were observed to be greater than those against extra-cellular bacteria 4. Therefore, the authors of that report questioned the suggestions by other authors 1 about the accumulation of rifamycins within macrophages. It is conceivable that rifamycins may accumulate within macrophages but lack some mechanisms of activity under unfavorable intracellular conditions. In the present study, the MICs of Clarithromycin and rifabutin either in combination or alone against intracellular bacteria were no different from those against extracellular bacteria, which confirms previous conclusions that one cannot always anticipate the increased levels of activity of these agents against the bacteria residing in macrophages just because of their possible intracellular accumulation.
Prolonged exposure of infected macrophages to drugs for 4 weeks resulted in the same MICs of each drug as those found during the 1-week exposure. A slight decrease in the number of viable bacteria occurred in some experiments at weeks 3 through 4 or at week 4 in the presence of one time the MIC of Clarithromycin alone or in a combination of Clarithromycin and rifabutin (one-half the MICs of each drug). At the same time, a twofold increase (each drug at one time the MIC) resulted in a substantial reduction (about 100-fold) in the number of viable bacteria during this prolonged period of exposure. These experiments have shown the possibility that rifabutin may enhance the activity of Clarithromycin against intracellular bacteria. Finally, 2-h pulsed exposures of the infected macrophages to Clarithromycin and rifabutin concentrations attainable at the time of Cmax have indicated that the effect of the combination was mainly due to Clarithromycin and it was only inhibitory, without any substantial reduction in the number of viable bacteria.
In conclusion, the combination of Clarithromycin and rifabutin produced additive effects against both extra- and intracellular bacteria, mainly inhibitory, with the potential for enhanced activity during prolonged periods of exposure to the drugs.
This study was supported by Abbott Laboratories. We thank L. Landskroner for the illustrations and C. Queen for preparation of the manuscript.
| 1. | Acocella, G., N. A, Carione, A, M. Cuffini, and G. Cavalla. 1985. The penetration of rifampicin, pyrazinamide, pyrazinoic acid into macrophages. Am. Rev. Respir. Dis. 132:1268-1273. |
| 2. | Baradelli, L. B., G. L Plosker, and D. McTavish. 1993. Clarithromycin. A review of its pharmacological properties and therapeutic use in Mycobacterium avium-intracellurare complex infection in patients with acquired immune deficiency syndrome. Drugs 46:289-312. |
| 3. | Brown, S. T., F. F. Edwards, E. M. Bernard, and D. Armstrong. 1991. Inhibition of Mycobacterium avium complex (MAC) by single agents and combinations. Program Abstr. 31st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 674. |
| 4. | Dhillon, J., and D. A, Mitchison. 1992. Activity in vitro of rifabutin, FCE 22807, rifapentine, and rifampin against Mycobacterium microti and M tuberculosis and their penetration into mouse peritoneal macrophages. Am. Rev. Respir. Dis. 145:212-214. |
| 5. | Heifets, L. B. 1991. Drug combinations, p. 179-200. In L. B. Heifets (ed.), Drug susceptibility in the chemotherapy of mycobacterial infections. CRC Press, Inc., Boca Raton, Fla. |
| 6. | Heifets, L. B., M.D. Iseman, P. J. Lindholm-Levy, and W. Kanes. 1985. Determination of ansamycin MICs for Mycobacterium avium complex in liquid medium by radiometric and conventional methods. Antimicrob. Agents Chemother. 28:570-575. |
| 7. | Heifets, L. B., P. J. Lindholm-Levy, and R, D. Comstock. 1992. Bacteriostatic and bactericidal activities of gentamicin alone and in combination with Clarithromycin against Mycobacterium avium. Antimicrob. Agents Chemother. 36:1695-1698. |
| 8. | Heifets, L. B., P. J. Lindholm-Levy, and L D. Comstock. 1992. Clarithromycin minimal inhibitory and bactericidal concentrations. Am. Rev. Respir. Dis. 145:856-858. |
| 9. | Ji, B., N. Lounis, C. Trufot-Pernot, and J. H. Grosset 1992. In vitro activities of clarithromycin-containing double- or triple-drug combinations against Mycobacterium avium complex First Int Conf. Macrolides, Azalides and Streptogramins. |
| 10. | Kent, R. J., M. Bakhtiar, and D.C. Shanson. 1992. The in vitro bactericidal activities of combinations of antimicrobial agents against clinical isolates of Mycobactertum avium-intracellulare, Antimicrob. Chemother. 30:643-650. |
| 11. | Kroogstad, D. J., and R. C. Moellering. 1986. Antimicrobial combinations, p. 537-560. In V. Lorian (ed.), Antibiotics in laboratory medicine, 2nd ed. The Williams & Wilkins Co, Baltimore. |
| 12. | Mor, N., and L. Heifets. 1993. MIC and MBC of clarithromycin against Mycobactenum avium within human macrophages Antimicrob. Agents Chemother. 37:111-114. |
| 13. | Mor, N., and L. Heifets. 1993. Inhibition of intracellular growth of M. avium by one pulsed exposure of infected macrophages to clarithromycin. Antimicrob. Agents Chemother. 37:1380-1382 |
| 14. | Mor, N., J. Vanderkolk, and L. Heifets. 1994. Accumulation of Clarithromycin in macrophages infected with M. avium Pharmacotherapy 14:100-104. |
| 15. | Mor, N., J. Vanderkolk, and L. Heifets. 1994. Inhibitory and bactericidal activities of levofloxacin against Mycobacterium tuberculosis in vitro and in human macrophages. Antimicrob. Agents Chemother. 38:1161-1164. |
| 16. | Rastogi, N., and V. Labrousse. 1991. Extracellular and intrcellullar activities of clarithromycin used alone and in association with ethambutol and rifampin against Mycobacterium avium complex. Antimicrob. Agents Chemother. 35:462-470. |
| 17. | Rastogi, N., V. Labrousse, K. S. Goh, and J. P.C. DeSousa 1991. Antimycobacterial spectrum of sparfloxacin and its activities alone and in association with other drugs against Mycobacterium avium complex growing extracellularly and intracellularly in murine and human macrophages. Antimicrob. Agents Chemother. 35:2473-2480. |
* Corresponding author.
National Jewish Center for Immunology and Respiratory Medicine,
1400 Jackson St.,
Denver, CO 80206.
USA.
Phone: (303) 398-1384.
Fax: (303) 398-1953.
| Minimum Inhibitory Concentration (µg/ml) | |||||
| Strain | Singly | In combination | FIC | ||
| Clarithromycin | Rifabutin | Clarithromycin | Clarithromycin | ||
| 453 | 0.5 | 0.25 | 0.25 | 0.125 | 1.0 |
| 3010 | 0.5 | 0.25 | 0.25 | 0.25 | 1.5 |
| 3354 | 1.0 | 0.5 | 1.0 | 0.5 | 2.0 |
| Minimum Inhibitory Concentration (µg/ml) | ||||
| Strain | Singly | In combination | ||
| Clarithromycin | Rifabutin | Clarithromycin | Clarithromycin | |
| 453 | 1.0 | 0.5 | 0.5 | 0.25 |
| 3010 | 1.0 | 0.5 | 0.5 | 0.25 |
| 3354 | 1.0 | 0.5 | 0.5 | 0.25 |
aThe FIC was 1 for all strains.
| Minimum Bactericidal Concentration (µg/ml) | ||||
| Strain | Singly | In combination | ||
| Clarithromycin | Rifabutin | Clarithromycin | Clarithromycin | |
| 453 | 64.0 | 8.0 | 32.0 | 4.0 |
| 3010 | 32.0 | 8.0 | 32.0 | 4.0 |
| 3354 | 64.0 | 4.0 | 32.0 | 2.0 |
aThe FBC was 1 for all strains.