This site is no longer maintained and is presented for archive purposes only
| 1 |
Department of Food Science (Food Microbiology), The Queen's University of Belfast, Newforge Lane, Belfast BT9 5PX, Northern Ireland, UK. |
2 |
Veterinary Sciences Division, Department of Agriculture for Northern Ireland, Stoney Road, Belfast BT4 3SD, Northern Ireland, UK. |
The efficacy of high-temperature, short-time (HTST) pasteurisation (72oC/15 s) when low numbers (<= 103 CFU/ml) of Mycobacterium paratuberculosis are present in milk was investigated. Raw cows' milk spiked with Myco. paratuberculosis (103 CFU/ml, 102 CFU/ml, 10 CFU/ml, and 10 CFU/50ml) was subjected to HTST pasteurisation using laboratory pasteurising units. Ten bovine strains of Myco. paratuberculosis were tested in triplicate. Culture in BACTEC Middlebrook 12B radiometric medium detected acid-fast survivors in 14.8% and 10% of HTST-pasteurised milk samples at the 103 and 102 CFU/ml inoculum levels, respectively, whereas conventional culture on Herrold's egg yolk medium containing mycobactin J detected acid-fast survivors in only 3.7% and 6.7% of the same milk samples. IS900-based PCR confirmed that these acid-fast survivors were Myco. paratuberculosis. No viable Myco. paratuberculosis were isolated from HTST-pasteurised milk initially containing either 10 CFU/ml or 10 CFU/50 ml.
Mycobacterium paratuberculosis causes paratuberculosis, commonly known as Johne's disease (JD), in cattle, sheep, goats and other ruminants (Cocito et al. 1994). Although not currently classified as a zoonotic agent, Myco. paratuberculosis has been identified in intestinal biopsy tissue from a proportion of patients with Crohn's disease (CD) (Chiodini 1989). Whether this indicates a causative role for Myco. paratuberculosis in CD, or simply a complicating infection, is still the subject of much debate. However, if Myco. paratuberculosis is implicated in CD, then milk has been suggested as a possible vehicle of transmission of the organism from cattle to humans (Hermon-Taylor 1993; Thompson 1994). Detectable quantities of Myco. paratuberculosis have been reported in the milk of both clinically affected (Taylor et al. 1981) and asymptomatic cattle (Streeter et al. 1995; Sweeney et al. 1992) with JD. Taylor et al. (1981) cultured Myco. paratuberculosis from milk of 9 of 26 (35%) cows confirmed to have advanced JD. More recently, in the examination of milk from asymptomatic cows, Sweeney et al. (1992) cultured the organism from 9 of 77 (11.6%) milk samples and reported a titre of 2-8 CFU per 50 ml of milk, and Streeter et al. (1995) cultured the organism from 3 of 126 (2.3%) milk samples. The study by Sweeney et al. (1992) suggests a low incidence of Myco. paratuberculosis in the milk of naturally infected cattle, at least in asymptomatic animals.
There has been increased interest in the efficacy of current milk pasteurisation conditions as a result of the above findings, and the possible association of Myco. paratuberculosis with Crohn's disease. Two recent studies (Chiodini and Hermon-Taylor 1993; Grant et al. 1996), which investigated the efficacy of holder (63.5oC/30 min) and high-temperature, short-time (HTST, 71.7oC/15 s) pasteurisation when high numbers (104 and 107 CFU/ml) of Myco. paratuberculosis were present in milk, showed that neither holder nor HTST pasteurisation completely inactivated Myco. paratuberculosis when the organism was present at levels >= 104 CFU/ml. The numbers of Myco. paratuberculosis added to the milk in these pasteurisation studies were probably unrealistically high and unlikely to be encountered in naturally-infected milk which is to be pasteurised. However, the use of such high numbers of M. paratuberculosis in these studies was fully justified. Published guidelines on the design of thermal inactivation studies (Brown 1991; McClure et al. 1994) recommend that inoculum levels as high as 108-109 CFU/ml are employed in order to obtain a reliable measure of the heat sensitivity of an organism.
The study reported here was undertaken to determine whether HTST pasteurisation would completely inactivate Myco. paratuberculosis when lower numbers (103 CFU/ml, 102 CFU/ml, 10 CFU/ml or 10 CFU/50ml) of the organism were present in milk. The lowest of these inoculum levels was chosen to simulate the numbers of Myco. paratuberculosis recorded in the milk of asymptomatic cattle by Sweeney et al. (1992). The findings of this study supplement those of our previous study (Grant et al. 1996).
Ten bovine strains of Myco. paratuberculosis, described previously (Grant et al. 1996), were included in this study.
Raw milk was aseptically obtained from a healthy Friesian cow as decribed previously (Grant et al. 1996). Briefly, the udder was thoroughly cleaned with a warm disinfectant solution, rinsed with sterile distilled water and dried with a sterile udder cloth. The first few discharges of milk from each teat were discarded before milk samples were hand-milked into 300ml sterile bottles. The milk was transported to the laboratory on ice and then refrigerated overnight at 4oC. Prior to use the raw milk was routinely tested for the presence of antibiotics and a total viable count (TVC) at 30oC was determined by standard methods.
A standard suspension of Myco. paratuberculosis cells was prepared by washing growth from slopes of Herrold's egg yolk medium (HEYM) with phosphate buffered saline (PBS; pH 7.4), centrifuging at 2,500 x g for 20 min, and resuspending the pellet in sufficient PBS to yield a suspension containing approximately 108 Myco. paratuberculosis CFU/ml (determined by nephelometry). One millilitre of an appropriate dilution (10-3, 10-4, 10-5 or 10-7) of this standard suspension was added to 250 ml aliquots of raw milk to yield inoculated milk samples containing approximately 103 CFU/ml, 102 CFU/ml, 10 CFU/ml or 10 CFU/50 ml, respectively.
The laboratory-scale pasteurising apparatus and method used to simulate HTST pasteurisation (71.7oC/15 s) in the laboratory have been described previously (Franklin 1965; Grant et al. 1996). Briefly, the HTST pasteurising unit was placed in a water bath (Grant Type SB3) operating at 72±0.1oC and allowed to equilibrate to temperature. Inoculated milk (250 ml) was poured into the unit via the inlet funnel and heated for a total of 70 sec (55 sec 'come-up' time + 15 sec holding time). The entire apparatus was then transferred to a circulating cold water bath operating at 6oC. Once the pasteurised milk had cooled to below 8oC it was transferred, via the outlet spout, to a sterile screw-capped container and held at 4oC until sampled.
All ten strains of Myco. paratuberculosis were subjected to HTST pasteurisation on three separate occasions at each of the four inoculum levels.
A 3-slope tube dilution method was used to enumerate viable Myco. paratuberculosis in each milk sample before and after pasteurisation. Where necessary, low numbers of the organism were concentrated prior to enumeration by centrifuging (2,500 x g for 15 min) 10 or 50 ml aliquots of milk and resuspending the pellet of cells in 1 ml PBS. Milk samples were vigorously mixed and diluted as necessary. Aliquots (100µl per HEYM slope and 500µl per BACTEC vial) of undiluted milk, or appropriate dilutions or concentrations of it, were inoculated onto HEYM slopes containing 2 µg/ml mycobactin J (three slopes per dilution and at least three dilutions per milk sample) and into BACTEC Middlebrook 12B radiometric medium (Becton Dickinson UK Ltd., Cowley, Oxford) supplemented with 2 µg/ml mycobactin J and 0.5 ml sterile egg yolk emulsion (Difco). Both media were incubated at 37oC for up to 18 weeks. HEYM slopes were visually examined periodically for the presence of typical colonies and the mean number of Myco. paratuberculosis present per ml of milk was determined by Bayesian analysis of the tube dilution data as described by Roussanov et al. (1996). BACTEC vials were read every two weeks using a BACTEC 460 instrument. A growth index (GI) reading > 30 was considered presumptively positive. Confirmation that any growth observed was acid-fast was obtained by Ziehl-Neelsen staining.
In order to verify the viability of suspect survivors, and provide pure cultures for PCR confirmation, HEYM and BACTEC cultures from pasteurised milk samples suspected of containing viable Myco. paratuberculosis cells were decontaminated, by treatment with 4% (w/v) NaOH at 37oC for 15 min followed by neutralization with 14% (w/v) KH2PO4, prior to sub-culture onto fresh HEYM slopes and BACTEC media.
In the case of HEYM slope cultures one colony was picked off and suspended in 50µl of sterile deionized water, vortexed for 1 min and the suspension heated to 100oC for 15 min to release the DNA. BACTEC cultures were prepared for PCR testing as described by Cousins et al. (1995). Briefly, suspect BACTEC cultures were sub-cultured into fresh BACTEC 12B medium containing mycobactin J but no egg yolk, and incubated until the GI had reached >= 50, when 100 µl was removed, heated to 94oC for 10 min in order to lyse cells and release DNA. For PCR amplification 5µl of lysed DNA from either HEYM or BACTEC culture was added to 45µl aliquots of PCR mix, consisting of 50mM KCl, 10mM Tris-HCl, pH 9.0 and 0.1% Triton X-100, 1.75mM MgCl2, 150µM of each dNTP, 60 pmol each of oligonucleotide primers P90 and P91 (Moss et al. 1992) and 1.25 units of Taq DNA polymerase, and overlaid with 2 drops of light mineral oil. PCR amplification was carried out on a thermal cycler (Perkin-Elmer). The amplification cycle consisted of an initial denaturation of target DNA at 94oC for 4 min, followed by 40 cycles of denaturation at 94oC for 1 min, primer annealing at 58oC for 2 min and primer extension at 72oC for 2 min. The final cycle included extension at 72oC for 7 min and refrigeration at 4oC. Primers P90 and P91, previously described by Moss et al. (1992), were selected to amplify a unique 400 base-pair (bp) fragment of the 5' region of the insertion element IS900 which is specific to Myco. paratuberculosis. Negative (water only) and positive (Myco. paratuberculosis DNA) PCR controls were run in parallel with each series of PCR samples. PCR products were visualized on 2% (w/v) agarose gels stained with ethidium bromide (0.5µg ml-1) and the sizes of amplified products determined by utilizing (FX174 RF DNA/Hae III molecular weight markers (Sigma).
The mean TVC of the raw milk used in this study was 580 CFU/ml and all raw milk used contained < 0.003 IU of penicillin per ml. The mean number of Myco. paratuberculosis present in the raw milk samples after spiking at each of the inoculum levels is shown in Table 1. These values indicate that the inocula achieved were marginally lower than intended.
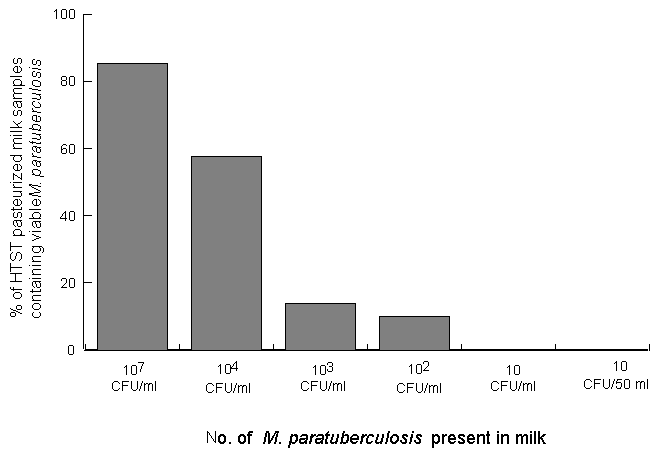
This study was carried out to determine whether HTST pasteurisation (72oC for 15 s) is effective in killing Myco. paratuberculosis when the organism is present in milk in low numbers. All laboratory-pasteurised milk samples tested negative by the phosphatase test, indicating that adequate pasteurisation had occurred. A small number of viable acid-fast survivors were isolated from both HEYM and BACTEC cultures of pasteurised milk samples initially containing 103 or 102 CFU of Myco. paratuberculosis/ml, but no survivors were isolated from any of the pasteurised milk samples initially containing either 10 CFU/ml or 10 CFU/50ml. BACTEC culture detected acid-fast survivors in 4 of 27 (14.8%) and 3 of 30 (10%) HTST-pasteurised milk samples at the 103 and 102 inoculum levels respectively, whereas survivors were detected in only 1 of 27 (3.7%) and 2 of 30 (6.7%) of the same milk samples by HEYM culture. Following checks to ensure that these acid-fast survivors were viable, a total of 10 viable cultures (3 from HEYM and 7 from BACTEC ) from 114 HTST-pasteurised milk samples were confirmed to be Myco. paratuberculosis using IS900-based PCR. The mean number of Myco. paratuberculosis cells surviving HTST pasteurisation of milk was found to be 6.3 CFU/ml when 103 CFU of Myco. paratuberculosis/ml were present initially, and 7.0 CFU/10 ml when 102 CFU of Myco. paratuberculosis/ml were present (Table 1). Our previous study (Grant et al. 1996) showed that when 107 and 104 CFU/ml were initially present in the milk 85.3% and 57.6% of HTST pasteurised milk samples, respectively, contained surviving Myco. paratuberculosis. The results of this further study show that the percentage of milk samples containing viable Myco. paratuberculosis after HTST pasteurisation continued to decrease as the number of Myco. paratuberculosis initially present decreased (Fig.1). However, HTST pasteurisation was only completely effective when < 10 CFU of Myco. paratuberculosis/ml were present in the milk.
Some variation in heat sensitivity between strains of Myco. paratuberculosis was observed in the course of this study. For example, strains ATCC 19698, B4, DVL 940 and USDA 1113 were found to be more heat sensitive and did not survive any of the pasteurisation treatments, whereas the other six strains were represented among the survivors.
Radiometric culture proved to be a more sensitive method of isolating viable Myco. paratuberculosis from pasteurised milk than conventional culture on HEYM. Overall, three and seven pasteurised milk samples containing viable Myco. paratuberculosis were detected by HEYM and BACTEC culture respectively. This is in agreement with previous reports that BACTEC culture is more sensitive than conventional culture on HEYM for the isolation of Myco. paratuberculosis from faecal and clinical samples (Collins et al. 1988; Sockett et al. 1992). The greater detection rate may simply be due to the fact that a greater volume of inoculum was added to each BACTEC vial (500 µl) than was added to each HEYM slope (100 µl). Alternatively, it may suggest that liquid culture is more conducive to the recovery of surviving, possibly sub-lethally injured, Myco. paratuberculosis than culture on solid medium. If only conventional culture on HEYM had been used to detect survivors after HTST pasteurisation in this study the number of milk samples containing viable Myco. paratuberculosis would have been considerably underestimated. This clearly highlights the importance of using suitable media to isolate Myco. paratuberculosis cells which may be sub-lethally injured as a result of pasteurisation but able to recover under suitable conditions.
The main finding of this study was that Myco. paratuberculosis may survive HTST pasteurisation if present in milk at levels of 103 and 102 CFU/ml prior to heat treatment, but will be completely inactivated by HTST-pasteurisation when levels ( 10 CFU/ml are present. These results were rather unexpected since milk pasteurisation could be expected to achieve the complete inactivation of such low numbers of microoganisms. Therefore, the question of how closely our laboratory-scale HTST pasteurising units simulate commercial HTST pasteurisation must be addressed. The major difference between pasteurisation in these laboratory-scale pasteurising units and in a commercial pasteuriser is that milk would be in continuous motion in the latter, but static in the former. Franklin (1965), in his original paper describing the development of the laboratory-scale HTST pasteurising units used in the present study, addressed this question. He concluded that providing a laboratory apparatus closely mimiced similar rates of heating and cooling, as well as holding time, and resulted in similar phosphatase destructions to those obtained with commercial plant over a range of temperatures, then it was reasonable to assume that the two methods were equivalent. The heating/cooling profiles of the HTST pasteurising units employed during this study were published previously (Grant et al. 1996), and closely resemble those published by Franklin (1965), and more recently by Hinrichs and Kessler (1995), for a commercial HTST pasteurising plant. Franklin (1965) also concluded that it was not essential that a laboratory method should be of a continuous flow type, since a batch process would yield a product composed of particles of milk, and presumably microorganisms, which had been heated to slightly differing extents but which had all received the minimum heat treatment for milk pasteurisation (i.e. 72oC for 15 s). We have temperature data to confirm that milk achieved this minimum heat treatment in each of the pasteurisation trials carried out in the course of this study and, therefore, we are confident that the results we have presented are reliable.
The funding for this work was provided by the Ministry of Agriculture, Fisheries and Food, United Kingdom.
Brown, W.L. (1991) Designing Listeria monocytogenes thermal inactivation studies for extended-shelf-life refrigerated foods. Food Technology 45 (4), 152-153.
Chiodini, R.J. (1989) Crohn's disease and the mycobacterioses: a review and comparison of two disease entities. Clinical Microbiology Reviews 2, 90-117.
Chiodini, R.J. and Hermon-Taylor, J. (1993) The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurisation. Journal of Veterinary Diagnostic Investigations 5, 629-631.
Cocito, C., Gilot, P., Coene, M., De Kesel, M., Poupart, P. and Vannuffel, P. (1994) Paratuberculosis. Clinical Microbiology Reviews 7, 328-345.
Collins, M.T., Lambrecht, R.S. and McDonald, J. (1988) Radiometric detection of Mycobacterium paratuberculosis from clinical specimens. In Proceedings of the Second International Colloquium on Paratuberculosis. ed. Merkal, R.S. and Thorel, M.F. pp.179-188. Maisons-Alfort, France: Laboratoire Central de Recherches.
Cousins, D.V., Evans, R.J. and Francis, B.R. (1995) Use of BACTEC radiometric culture method and polymerase chain reaction for the rapid screening of faeces and tissues for Mycobacterium paratuberculosis. Australian Veterinary Journal 72, 458-462.
Franklin, J.G. (1965) A simple laboratory-scale HTST milk pasteuriser. Journal of Dairy Research 32, 281-289.
Grant, I.R., Ball, H.J., Neill, S.D. and Rowe, M.T. (1996) Inactivation of Mycobacterium paratuberculosis in cow's milk at pasteurisation temperatures. Applied and Environmental Microbiology 62, 631-636.
Hermon-Taylor, J. (1993) Causation of Crohn's disease: the impact of clusters. Gastroenterology 104, 643-646.
Hinrichs, J. and Kessler, A.G. (1995) Thermal processing of milk - processes and equipment. In Heat-induced changes in milk, 2nd edition. ed. Fox P.F. pp. 9-21. Brussels: International Dairy Federation.
McClure, P.J., Blackburn, C. de W., Cole, M.B., Curtis, P.S., Jones, J.E., Legan, J.D., Ogden, I.D., Peck, M.W., Roberts, T.A., Sutherland, J.P. and Walker, S.J. (1994) Modelling the growth, survival and death of microorganisms in foods: the UK Food Micromodel approach. International Journal of Food Microbiology 23, 265-275.
Moss, M.T., Sanderson, J.D., Tizard, M.L.V., Hermon-Taylor, J., El-Zaatari, F.A.K., Markesich, D.C. and Graham, D.Y. (1992) Polymerase chain reaction detection of Mycobacterium paratuberculosis and Mycobacterium avium subsp. silvaticum in long term cultures from Crohn's disease and control tissues. Gut 33, 1209-1213.
Roussanov, B., Hawkins, D.M. and Tatini, S.R. (1996) Estimating bacterial density from tube dilution data by a Bayesian method. Food Microbiology 13, 341-363.
Sockett, D.C., Carr, D.J. and Collins, M.T. (1992) Evaluation of conventional and radiometric fecal culture and a commercial DNA probe for diagnosis of Mycobacterium paratuberculosis infections in cattle. Canadian Journal of Veterinary Research 56, 148-153.
Streeter, R.N., Hoffsis, G.F., Bech-Nielsen, S., Shulaw, W.P. and Rings, M. (1995) Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. American Journal of Veterinary Research 56, 1322-1324.
Sweeney, R.W., Whitlock, R.H. and Rosenberger, A.E. (1992) Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. Journal of Clinical Microbiology 30, 166-171.
Taylor, T.K., Wilks, C.R. and McQueen, D.S. (1981) Isolation of Mycobacterium paratuberculosis from the milk of a cow with Johne's disease. Veterinary Record 109, 532-533.
Thompson, D.E. (1994) The role of mycobacteria in Crohn's disease. Journal of Medical Microbiology 41, 74-94.

| Intended inoculum level |
Number of viable Myco. paratuberculosis present (Mean ± S.D.) |
|
| Before pasteurisation | After pasteurisation | |
| 103 CFU/ml | 468.0 ± 1.6 CFU/ml | 6.3 ± 2.5 CFU/ml |
| 102 CFU/ml | 50.0 ± 2.0 CFU/ml | 7.0 CFU/10ml |
| 10 CFU/ml | 6.3 ± 1.2 CFU/ml | ND* |
| 10 CFU/50ml | 2.0 ± 0.8 CFU/50ml | ND |
* No viable Myco. paratuberculosis detected in 50 ml of pasteurised milk.