This site is no longer maintained and is presented for archive purposes only
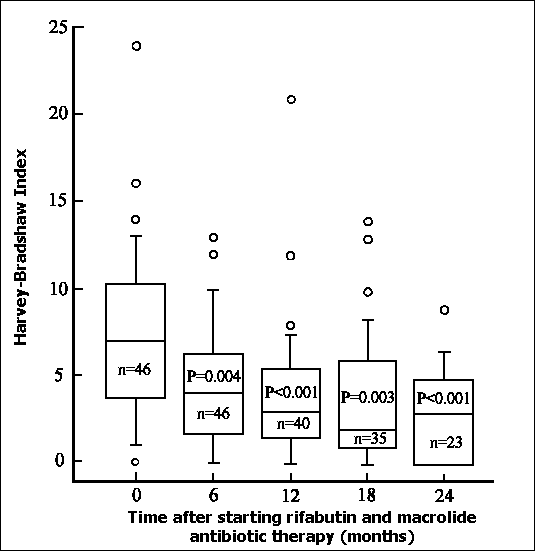
Fifty-two patients with severe Crohn's disease were enrolled in this study. Six (11.5%) were intolerant of the medication and had to be excluded. The remaining 46 patients were treated with rifabutin in combination with a macrolide antibiotic(clarithromycin or azithromycin). Patients were treated for a mean of 18.7 (range 6-35) months and followed up for 25.1 (range 7-41) months. Of the 19 patients who were steroid dependent at the start of this study, only two continued to require steroids when treatment was established. A reduction in the Harvey-Bradshaw Crohn's disease activity index occurred after 6 months treatment (P = 0.004, paired Wilcoxon test) and was maintained at 24 months (P < 0.001). An improvement in inflammatory parameters was observed as measured by a reduction in erythrocyte sedimentation rate (P = 0.009) and C-reactive protein (P = 0.03) at 18 months compared with pretreatment levels, and an increase in serum albumin at 12 months (P = 0.04). When subsets of the study population were analyzed, patients with pan-intestinal disease achieved better remission at 2 years than did those with less extensive involvement (P = 0.04, Mann-Whitney U-test). No difference in treatment response by age, disease duration, the presence of granulomas on histology, or the occurrence of drug-induced side-effects was observed. These data suggest that treatment with rifabutin and clarithromycin may result in a substantial clinical improvement in Crohn's disease and justify the conduct of a randomized controlled trial.
While the cause of Crohn's disease remains controversial , evidence for the involvement of Mycobacterium paratuberculosis has been accumulating from both long-term culture1-8 and polymerase chain reaction (PCR) tests on diseased tissue 9-15. M. paratuberculosis is a specific chronic enteric pathogen capable of affecting many animal species including primates 16,17. Recent evidence suggests that this organism may be conveyed to humans in pasteurized cows' milk 18. Although case reports and a small open study using antituberculous drugs to treat Crohn's disease have shown promising results 19,20, the efficacy of this approach has never been confirmed in randomized controlled trials 21-23. M. paratuberculosis, like other Mycobacterium avium, is generally resistant to standard antituberculous drugs, and in-vivo infections are known to be difficult to eradicate 24. Rifabutin, clarithromycin and azithromycin are a new generation of antibiotics which may have enhanced activity against M. paratuberculosis 25,28. This paper presents an outcomes analysis of the efficacy of these drugs used in combination for the treatment of Crohn's disease.
Fifty-two patients with Crohn's disease, most of whom had persistent severe symptoms refractory to conventional treatment, were studied. Six patients had to be excluded, one due to acute uveitis and five due to intolerance of the medication comprising general malaise , lethargy, arthralgia, nausea and vomiting. The evaluable study population therefore consisted of 46 patients. The details of patients at entry to the study are given in the Table. Forty-two (91.3%) patients were selected for rifabutin and macrolide antibiotic therapy (RMAT) as they were no longer responsive to standard medical therapy. Seven (15.2%) patients had been on steroids alone, 19 (41.3%) patients had taken steroids and sulphasalazine or mesalazine, four patients (8.7%) steroids and azathioprine, seven (15.2%) patients steroids, azathioprine and sulphasalazine or mesalazine, and five (10.9%) patients sulphasalazine or mesalazine alone. Nineteen patients were steroid dependent at the start of RMAT. Four (8.7%) new patients were started directly on RMAT because of extensive active Crohn's disease. Previous surgery had been undertaken before commencing RMAT in 26 (46.5%) of the patients. The duration of the disease, and extensive previous drug treatment and surgery, reflect the usual clinical pattern seen in patients referred to tertiary centers specializing in the management of inflammatory bowel disease.
Patients were included in the study if they had an established diagnosis of active Crohn's disease on clinical, radiological (barium studies and or technetium-99 white-cell scan) and histopathological grounds. Granulomas were identified in the disease tissue sections of 60% of patients. The RMAT regimen consisted of rifabutin 300mg in the morning and 150mg at night (Mycobutin, Pharmacia Ltd, Milton Keynes, UK) and either clarithromycin 250mg twice daily (Klaricid, Abbott Laboratories Ltd, Queensborough, UK) or azithromycin 500mg in a single daily dose for 4 consecutive days each week (Zithromax, Pfizer Ltd, Sandwich, UK). Forty-three patients were treated with a combination of rifabutin and clarithromycin of whom ten had also received a quinolone (either ciprofloxacin 500mg twice daily (Bayer plc, Newbury, UK) or ofloxacin 200mg twice daily (Roussel Laboratories Ltd, Uxbridge, UK)). Five patients received clofazamine 100mg once daily (Geigy Pharmaceuticals, Horsham, UK) in addition to rifabutin and clarithromycin. Three patients were treated with rifabutin and azithromycin alone. Patients received RMAT for a mean duration of 18.7 months (median 18 months, range 6-35 months).
The mean follow-up time after commencing RMAT was 25.1 months (median 28 months, range 8-41 months). Data were extracted from the hospital records with the following outcome measures as end-points: Harvey-Bradshaw disease activity index 29; haemoglobin , white blood cell and platelet counts; erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and albumin; body mass index; liver biochemistry profile; steroid dependency and the need for surgery during or after commencing RMAT. Each of these parameters was analyzed at 6 month intervals for up to two years and compared with pre-treatment values using the paired Wilcoxon test. The number of patients within each time frame after starting RMAT were: 6 months, n = 46; 12 months, n = 45; 18 months, n = 41; and 24 months, n = 29. White cell scans were performed after completion of RMAT in 11 patients in whom pretreatment images were also available for comparison.
The Cox proportional hazard survival analysis model was used to evaluate the maintenance of remission during and after RMAT. Subset analysis using the Mann-Whitney U-test was performed at 12 and 24 months to determine if there was a measurable difference in the Harvey-Bradshaw activity index in response to RMAT for each of the parameters.
Disease activity measured by the Harvey-Bradshaw index was significantly reduced within 6 months of commencing RMAT and was maintained for 24 months (figure 1). Clinical remission (Harvey-Bradshaw Activity Index < 5) was induced in 43 (93.5%) patients. Life table estimates of remission maintenance during and after treatment with RMAT derived by Cox proportional hazard survival analysis, are shown in figure 2. Of patients in whom a clinical remission was induced, the cumulative probability of remaining in remission after 24 months of RMAT was 68.7%.
The mean (median) ESR progressively fell from 18.5 (24) (range 5-118) mm/h pretreatment with RMAT, to 11.8 (11) (range 1-26) mm/h at 18 months (P = 0.009, paired Wilcoxon test). Correspondingly, the CRP fell from a mean (median) of 23.5 (8.3) (range 4.0-109.0) mg/L pretreatment, to 4.0 mg/L (4.0-50.3) at 18 months (P = 0.03). A significant increase in serum albumin compared with pretreatment value (Table) was observed at 12 and 18 months after commencement of RMAT, median value 40.0 g/L (P=0.05) and 40.5 g/L (P=0.04), respectively.
After 18 months of treatment with RMAT, there was a significant increase in the median haemoglobin concentration (16.1 g/dL, P=0.01) and a significant decrease in the platelet count (281.5 * 109/L, P=0.01) compared with pretreatment levels listed in the Table (paired Wilcoxon test). RMAT did not influence the median white blood cell count of the study population throughout the course of treatment.
RMAT had no overall effect over the period of study on either the body mass index or body weight in this cohort of patients.
The mean (median) concentration of the serum liver enzymes before RMAT were: alkaline phosphatase, 85.8 (68.5) (range 33-286) mmol/L; alanine transaminase, 17.3 (13.5) (range 4-54) mmol/L; bilirubin 8.5 (6.0) (range 2-65) micro-mol/L. RMAT had no significant effect on serum levels of alkaline phosphatase, alanine transaminase or bilirubin over the course of treatment.
At the time of commencing RMAT, 19 patients were steroid dependent, requiring a median cumulative dose of oral prednisolone over the preceding 6 months of 1575 g (range 81-5460 g). Steroid requirements over the period of study were significantly reduced when assessed by the paired Wilcoxon test: 6 months, median 445 g (range 0-3640 g), P<0.001; 12 months, median 0 (range 0-1820 g), P<0.001; 18 months, median 0 (range 0-5740 g), P=0.001; 24 months, median 0 (range 0-4550 g), P=0.007. Of the 19 patients who were on oral prednisolone at the time of starting RMAT, 12 were on steroids at 6 months and four at 12 months; only two patients remained steroid dependent at 2 years.
Thirty-one patients had been treated with a 5-amino salicylic acid derivative, and 11 patients had received azathioprine at some stage before starting RMAT. Of these, only three patients continued to require either sulphasalazine or mesalazine, and three patients required azathioprine during or at the end of treatment with RMAT.
A total of 15 (32.6%) patients required an operation during the course of RMAT. Indications for surgery were stricture formation in a segment of irreversibly damaged bowel in ten (66.7%) patients, fistula or abscess formation in three (20%) patients and active disease not controlled by medication in two (13.3%) patients. There were five (33.3%) ileal resections and/or strictureplasties, seven (46.7%) iloecaecal resections, two (13.3%) large bowel resections, and one laparotomy with biopsy only. To exclude the possibility that surgery itself might have been responsible for the improvement in the Harvey-Bradshaw activity index attributed to RMAT (figure 1), the disease activity analysis was repeated excluding the patients who had operations on treatment. The P-values by the paired Wilcoxon test compared with pre-treatment activity indices were P=0.03 at 6 months (n=32), P=0.002 at 12 months (n=28), P=0.01 at 18 months (n=23) and P=0.02 at 24 months (n=16). Although the scale of the clinical response appeared to be reduced by exclusion of the patients who underwent surgery on treatment, the overall significant improvement in disease activity was maintained up to 24 months.
Patients who had both small and large bowel disease appeared to do better than patients who had small bowel disease alone at 24 months, using median scores of the Harvey-Bradshaw activity index (P=0.04, Mann-Whitney U-test). Subsets analyzed by age (greater or less than 21 years), duration of disease (greater or less than 24 months from diagnosis), body mass index (greater or less than 20 kg/m2), the presence or absence of granulomas on histology, extra-gastrointestinal manifestations, the necessity for surgery during the study period, gender and smoking, revealed no significant difference in improvement as a result of RMAT at 12 and 24 months compared with pretreatment values. Likewise, there was no difference in clinical response to RMAT if patients were categorized according to whether surgery was required before treatment.
Eleven patients had white cell scans on completion of RMAT that could be compared with pretreatment scans. Six scans were unchanged, one showed continued activity but improvement after RMAT, and four showed complete remission with no evidence of residual active disease. An example of a white cell scan showing a major treatment response is illustrated in figure 3.
The commonest, yet non-distressing, side-effect was pseudojaundice, which caused yellow skin discoloration in 41 (89.1%) patients. Significant arthralgia was experienced by 23 (50%) patients. Four (8.7%) patients developed a maculopapular skin eruption, two (4.3%) suffered from dry eyes and there was a transient elevation of serum liver enzymes in one (2.2%) patient. Reversible rifabutin-induced acute uveitis occurred in four (8.7%) of the 46 patients studied. Two (4.3%) patients developed a transient neutropenia within a month of starting RMAT, with a white cell count of less than 3.0 * 109/L. While symptoms of general malaise usually resolved within the first three months of treatment, 18 (39.1%) patients continued to experience arthralgia beyond this time.
Previous randomized controlled trials to evaluate the use of a variety of antimycobacterial chemotherapeutic agents in Crohn's disease have not shown significant benefit in treatment compared with placebo controlled groups 21-23. Some of these trials may be criticized on the basis of single-agent therapy or the selection of inappropriate drugs, particularly for use against spheroplast forms of M. paratuberculosis in vivo. Combinations of quadruple therapy have been shown to be effective in the relief of symptoms and maintenance of remission in patients with active or steroid-dependent Crohn's disease 30,31. None of these trials evaluated a combination of rifabutin and the newer macrolide antibiotics, which are predicted to have greater activity against the specific chronic enteric pathogen M. paratuberculosis in vivo. Recent studies using this treatment regime in disseminated M. avium complex infections in patients with acquired immune deficiency syndrome have shown promising results 32-34.
Rifabutin is a synthetic rifamycin with greater bactericidal potential against M. paratuberculosis than rifampicin 26. In addition to the known effect on DNA-dependent RNA polymerase in bacteria, rifabutin has been shown to inhibit reverse transcriptase in HIV-1 35. Clarithromycin is also effective against M. paratuberculosis 28, and early reports using single-agent therapy demonstrated clinical efficacy in the treatment of active Crohn's disease in a randomized double blind crossover trial 36. The combination of rifabutin and clarithromycin has been shown to be additive against M. avium in vitro 37,38. The accumulation of the newer macrolide antibiotics including clarithromycin and azithromycin within human polymorphonuclear leucocytes and macrophages 25,39 may acccount for the enhanced efficacy against M. avium complex infections 40. The effects of the macrolide antibiotics alone or in combination may be potentiated by granulocyte-macrophage colony stimulating factor 27 or tumor necrosis factor 41.
The accurate assessment of activity in Crohn's disease is notoriously difficult 42. The Harvey-Bradshaw index represents a simplified yet reliable means of achieving this. Crohn's disease activity assessed by the Harvey-Bradshaw index was significantly reduced 6 months after commencement of RMAT, and maintained at 24 months. During this period, there was an objective improvement in the inflammatory markers (ESR, CRP and albumin), haematological parameters (haemoglobin and platelet count) and steroid requirements. Body weight is not a reliable marker of the overall presence or absence of clinical response in patients with Crohn's disease. Patients overweight due to steroid dependency at the start of RMAT, lost weight when they responded to RMAT enabling steroids to be withdrawn, whereas thin malnourished patients demonstrated a substantial weight gain in response to RMAT. To exclude the possibility that improvements in the Harvey-Bradshaw activity occurred because of surgical intervention during the RMAT treatment period, disease activity was reassessed with the patients who had operations excluded. Significant clinical improvement was still observed from 6 months after commencing RMAT and maintained until 24 months. Remission on RMAT was maintained in approximately two-thirds of the patients studied after two years.
Subset analysis of the study population did not identify any groups that were more likely to do well on RMAT with the exception of patients with both small and large bowel involvement, who achieved a better clinical response than patients with small bowel disease alone. This may be because clinical improvement in patients with extensive disease might have been easier to detect. Symptoms from Crohn's disease in humans arise from acute and chronic inflammation, superimposed in structural abnormalities such as strictures, fistulas and irreversibly damaged bowel. Variation in the exact cause of each patients symptoms, compounded by uncertainty in the interpretation of a finite therapeutic response on clinical, radiological or endoscopic grounds 43-45, might account for inconclusive results from small trials on the efficacy of RMAT in the treatment of Crohn's disease. Difficulty in culturing M. paratuberculosis and establishing growth in vitro precludes the establishment of strict culture-based microbiological criterion for disease eradication and cure. Future trials in Crohn's disease should monitor M. paratuberculosis in sequential endoscopic biopsies using hybridization capture of target DNA and IS900 PCR 46, together with the detection of IS900 RNA 15.
In conclusion, these data suggest that treatment with rifabutin in conjunction with a macrolide antibiotic is a safe combination that may induce and maintain remission as well as abolish steroid dependency in refractory Crohn's disease. As a proportion of patients with extensive Crohn's disease resistant to standard medical therapy respond to RMAT, further evaluation of this treatment as an additional therapeutic option is of great importance. These results justify a randomized, controlled trial to assess the efficacy of this therapeutic approach taking into account the standards for approval of new drugs for inflammatory bowel diseases 47.
| Male | n = 27 (58.7%) |
| Female | n = 19 (41.3%) |
| small bowel | n = 9 (19.6%) |
| large bowel | n = 14 (30.4%) |
| small and large bowel | n = 23 (50.0%) |
| perianal disease | n = 12 (26.1%) |
| extra-gastrointestinal | |
| -----arthralgia | n = 11 (23.9%) |
| -----eyes | n = 1 (2.2%) |
| -----skin | n = 1 (2.2%) |
| Smokers | n = 15 (32.6%) |
| mean | median | range | |
| Age at start of RMAT (years) | 30.2 | 27.5 | 13-73 |
| Duration from diagnoses (months) | 56.3 | 27.5 | 1-228 |
| Body mass index (kg/m2) | 21.1 | 21.5 | 13.7-32.9 |
| Haemoglobin (g/dL) | 12.6 | 12.5 | 10.1-16.3 |
| White cell count (*109/L) | 7.6 | 6.6 | 1-23.6 |
| Platelet count (*109/L) | 347 | 338 | 167-770 |
| Erythrocyte sedimentation rate | 24.2 | 17 | 5-118 |
| C-reactive protein | 23.5 | 8.3 | 4-109 |
| Albumin | 37.7 | 39.0 | 22-48 |
| Harvey-Bradshaw activity index | 7.9 | 7 | 0-36 |

Figure 1. Assessment of Crohn's disease using the Harvey-Bradshaw activity index at 6-monthly intervals after starting chemotherapy with rifabutin and clarithromycin or azithromycin. The number of patients in each cohort is shown, together with the significance values (Mann-Whitney U-test). The median is shown within each box, the 25th and 75th centiles form the short box edges, and the bars represent the 10th and 90th centiles. Points lying outside this range are shown as circles.
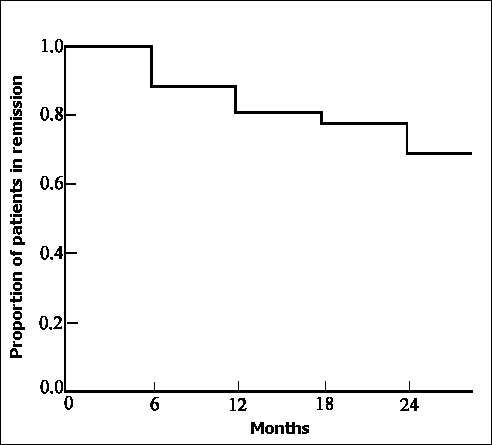
Figure 2. Life table estimate of remission maintenance in 43 patients with Crohn's disease during and after response to rifabutin and macrolide antibiotic therapy.

Figure 3. White cell scan of a 22 year old female patient (a) before combined treatment with rifabutin and clarithromycin showing (arrow) active inflammation in the diseased bowel, and (b) 22 months after treatment showing complete abolition of abnormal technetium-99 uptake. At this time the patient was, and remains, symptom free.
1. Chiodini, R.J., Van Kruiningen, H.J., Merkal, R.S., Thayer, W.R. and Coutu, J.A. (1984). Characteristics of an unclassified mycobacterium species isolated from patients with Crohn's disease. Journal of Clinical Microbiology 20, 966-71.
2. McFadden, J.J., Butcher, P.D., Chiodini, R. and Hermon-Taylor, J. (1987). Crohn's disease isolated mycobacteria are identical to Mycobacterium paratuberculosis as determined by DNA probes that distinguish mycobacterial species. Journal of Clinical Microbiology 25, 796-801.
3. Gitnick, G., Collins, J., Beaman, B., Brooks, D., Arthur, M., Imaeda, T., et al. (1989). Preliminary report on isolation of mycobacteria from patients with Crohn's disease. Digestive Diseases and Sciences 34, 925-32.
4. Thorel, M.F. (1989). Relationship between Mycobacterium avium, M. paratuberculosis and mycobacteria associated with Crohn's disease. Annales de Recherches Veterinaires 20, 417-29.
5. Hagsma, J., Mulder, C.J.J., Eger, A., and Tytgat, G.N.J. (1991) Mycobacterium paratuberculosis isole chez des patients atteints de Maladie de Crohn resultats preliminaires. Acta Endoscopica 21, 255-60.
6. Wall, S., Kunze, Z.M., Saboor, S., Soufleri, J., Seechurn, P., Chiodini, R., et al. (1993). Identification of Spheroplast-like agents isolated from tissues of patients with Crohn's disease and control tissues by Polymerase Chain Reaction. Journal of Clinical Microbiology 31, 1241-5.
7. Pavlic, I., Bejckova, L., Koskova, S., Fixa, B., Komarkova, O., and Bedrna, J. (1994). DNA fingerprinting as a tool for epidemiological studies of paratuberculosis in ruminants and Crohn's disease. In Proceedings of the Fourth International Colloquium on Paratuberculosis (Chiodini, R.J., Collins, M.T., Bassey, E., Eds), PP279-289, International Association for Paratuberculosis Inc., Reheboth, MA, USA.
8. Moss, M.T., Sanderson, J., Tizard, M., Hermon-Taylor, J., El-Zaatari, F., Markesich, D. et al. (1992). PCR detection of Mycobacterium paratuberculosis in long term cultures from Crohn's disease tissues. GUT 33, 1209-13.
9. Sanderson, J.D., Moss, M.T., Tizard, M.L.V., and Hermon-Taylor, J. (1992). Mycobacterium paratuberculosis DNA in Crohn's disease tissue. GUT 33, 890-6.
10. Dell'Isola, B., Poyart, C., Goulet, O., Mougenot, J.F., Sadoum-Journo, E., Brousse, N., et al. (1994). Detection of Mycobacterium paratuberculosis by Polymerase Chain Reaction in Children with Crohn's disease. Journal of Infectious Diseases 169, 449-51.
11. Fidler, H.M., Thurrell, W., Johnson, N.M., Rook, G.W., and McFadden, J.J. (1994). Specific detection of Mycobacterium paratuberculosis DNA associated with granulomatous tissue in Crohn's disease. GUT 35, 506-10.
12. Lisby, G., Andersen, J., Engbaek, K., and Binder, V. (1994). Mycobacterium paratuberculosis in intestinal tissue from patients with Crohn's disease demonstrated by a nested primer polymerase chain reaction. Scandinavian Journal of Gastroenterology 29, 923-9.
13. Erasmus, D.L., Victor, T.C., Van Eeden, P.J., Van Helden, P. (1995). Mycobacterium paratuberculosis and Crohn's disease. GUT 36, 942.
14. Suenaga, K., Yokoyama, Y., Okazaki, K., Yamamoto, Y. (1995). Mycobacteria in the intestine of Japanese patients with inflammatory bowel disease. American Journal of Gastroenterology 90, 76-80.
15. Mishina, D., Katsel, P., Brown, S.T., Gilberts, E.C.A.M., Greenstein, R.J., (1996). On the etiology of Crohn disease. Proceedings of the National Academy of Sciences of the United States of America 93, 9816-20.
16. Chiodini, R.J., Van Kruiningen, H.J., and Merkal, R.S. (1984). Ruminant paratuberculosis (Johne's disease): The current status and future prospects. Cornell Veterinarian 74, 218-62.
17. McClure, H.M., Chiodini, R.J., Anderson, D.C., Swenson, R.B., Thayer, W.R., Coutu, J.A. (1987). Mycobacterium paratuberculosis (Johne's disease) in a colony of stump-tail macaques(Macaca Arctoides). Journal of Infectious Diseases 155, 1011-9.
18. Millar, D., Ford, J., Sanderson, J., Withey, S., Tizard, M., Doran, T., et al. (1996). IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows milk in England and Wales. Applied and Environmental Microbiology 62, 3446-52.
19. Picciotto, A., Gesu, G.P., Schito, G.C., Testa, R., Varagona, G., Celle, G. (1988). Antimycobacterial chemotherapy in two cases of inflammatory bowel disease. Lancet II, 526-7.
20. Warren, J.B., Rees, H.C., Cox, T.M. (1986). Remission of Crohn's disease with tuberculosis chemotherapy. New England Journal of Medicine 314, 182.
21. Shaffer, J.L., Hughes, S., Linaker, B.D., Baker, R.D., Turnberg, L.A. (1984). Controlled trial of rifampicin and ethambutol in Crohn's disease. GUT 25, 203-5.
22. Afdhal, N.H., Long, A., Lennon, J., Crowe, J., O'Donoghue, D.P. (1991). Controlled trial of antimycobacterial therapy in Crohn's disease: clofazamine versus placebo. Digestive Diseases and Sciences 36, 449-53.
23. Swift, G.L., Srivasta, E.D., Stone, R., Pullan, R.D., Newcombe, R.G., Rhodes, J, et al. (1994). Controlled trial of anti-tuberculosis chemotherapy for two years in Crohn's disease. GUT 35, 363-8.
24. Gangadharam, P.R., Perumal, V.K., Jairam, B.T., Rao, P.N., Nguyen, A.K., Farhi, D.C., et al. (1987). Activity of rifabutin alone or in combination with clofazamine or ethambutol or both against acute and chronic experimental Mycobacterium intracellulare infections. American Review of Respiratory Disease 136, 329-33.
25. Panteix, G., Guillaumond, B., Harf, R., Desbos, A., Sapin, V., Leclercq, M., et al. (1993). In vitro concentration of azithromycin in human phagocytic cells. Journal of Antimicrobial Chemotherapy 31, suppl. E,1-4.
26. Luna-Herrera, J., Reddy, B., Gangadharam, P.R. (1995). In vitro and intracellular activity of rifabutin on drug-susceptible and multiple drug-resistant (MDR) tubercle bacilli. Journal of Anitmicrobial Chemotherapy 36, 355-63.
27. Onyeji, C.O., Nightingale, C.H., Tessier, P.R., Nicolau, D.P., Bow, L.M. (1995). Activities of clarithromycin, azithromycin, and ofloxacin in combination with liposomal or unencapsulated granulocyte-macrophage colony-stimulating factor against intra-microphage Mycobacterium avium-Mycobacterium intracellulare. Journal of Infectious Diseases 172, 810-6.
28. Rastogi, N., Goh, K.S., Labrousse, V. (1992). Activity of clarithromycin compared with those of other drugs against Mycobacterium paratuberculosis and further enhancement of its extra-cellular and intra-cellular activities by ethambutol. Antimicrobial Agents and Chemotherapy 36, 2843-6.
29. Harvey, R.F. and Bradshaw, J.M. (1980). A simple index of Crohn's-disease activity. Lancet II, 514.
30. Hampson, S.J., Parker, M.C., Saverymuttu, S.H., Joseph, A.E.A., McFadden, J.J., Hermon-Taylor, J. (1989). Quadruple anti-mycobacterial chemotherapy in Crohn's disease: Results at nine months of a pilot study in twenty patients. Alimentary Pharmacology and Therapeutics 3, 343-52.
31. Prantera, C., Kohn, A., Mangiarotti, R., Andreoli, A., Luzi, C. (1994). Anti-mycobacterial therapy in Crohn's disease: Results of a controlled, double-blind trial with a multiple antibiotic regimen. American Journal of Gastroenterology 89, 513-8.
32. Dautzenberg, B., Truffot, C., Legris, S., Meyohas, M.C., Berlie, H.C., Mercat, A., et al. (1991). Activity of clarithromycin against Mycobacterium avium infection in patients with the Acquired Immune Deficiency Syndrome. American Review of Respiratory Diseases 144, 564-9.
33. Young, L.S., Wiviott, L., Wu, M., Kolonoski, P., Bolan, R., Inderlide, C.B. (1991). Azithromycin for treatment of Mycobacterium avium-intracellulare complex infection in patients with AIDS. Lancet 338, 1107-9.
34. Nightingale, S.D, Cameron, D.W., Gordine, F.M., Sullam, P.M., Cohn, D.L., Chaisson, R.E., et al. (1993). Two controlled trials of rifabutin prophylaxis against Mycobacterium avium complex infection in AIDS. New England Journal of Medicine 329, 828-33.
35. Anand, R., Moore, J.L., Curren, J.W. & Srinivasan, A. (1988). Interaction between rifabutin and immunodeficiency virus type 1: Inhibition of replication cytopathic effect and reverse transcriptase in vitro. Antimicrobial Agents and Chemotherapy 32, 694-98.
36. Graham, D.Y., Al-Assi, M.T., and Robinson, M. (1995). Prolonged remission in Crohn's disease following therapy for Mycobacterium tuberculosis infection. Gastroenterology 108, A826.
37. Mor, N., Van Der Kolk, J., Mezo, N., and Heifets, L. (1994). Effects of clarithromycin and rifabutin alone and in combination on intracellular and extracellular replication of Mycobacterium avium. Antimicrobial Agents and Chemotherapy 38, 2738-42.
38. Fattorini, L., Li, B., Piersimoni, C., Tortoli, E., Xiao, Y., Santoro, C., et al. (1995). In vitro and ex vivo activities of antimicrobial agents used in combination with clarithromycin, with or without amikacin against Mycobacterium avium. Antimicrobial Agents and Chemotherapy 39, 680-5.
39. Fraschini, F., Scaglione, F., Pintucci, G., Maccarinelli, G., Dugnani, S., Demartini, G. (1991). The diffusion of clarithromycin and roxithromycin into nasal mucosa, tonsil, and lung in humans. Journal of Antimicrobial Chemotherapy 27, 61-5.
40. Bermudez, L.E., Martinelli, J., Petrofsky, M., Kolonoski, P., Young, L.S. (1994). Recombinant granulocyte-macrophage colony-stimulating factor enhances the effects of antibiotics against Mycobacterium avium complex infection in the beige mouse model. Journal of Infectious Diseases 169, 575-80.
41. Young L.S., Bermudez, L.E., Wu, M., and Inderlide, C.B. (1995). Potential role of roxithromycin against the Mycobacterium avium complex. Infection 23, suppl. 1, S28-32.
42. Hodgson, H.J.F. and Mazlan, M.Z. (1991). Review article: Assessment of drug therapy in inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 5, 555-84.
43. Modigliani, R., Mary, J.Y., Simon, J.F., Cortot, A., Soule, J.C., Gendre, J.P., et al. (1990). Clinical, biological and endoscopic picture of attacks of Crohn's disease. Gastroenterology 98, 811-8.
44. Olaison, G., Sjodahl, R., and Tagesson, C. (1990). Glucocorticoid treatment in ileal Crohn's disease: Relief of symptoms but not endoscopically viewed inflammation. GUT 31, 325-8.
45. Weldon, M.J., Lowe, C., Joseph, A.E.A., Maxwell, J.D. (1996). Review article: Quantitative leucocyte scanning in the assessment of inflammatory bowel disease activity and its response to therapy. Alimentary Pharmacology and Therapeutics 10, 123-32.
46. Millar, D.S., Withey, S.J., Tizard, M.L., Ford, J.G., Hermon-Taylor, J. (1995). Solid-phase hybridization capture of low abundance target DNA sequences: Application to the polymerase chain reaction detection of Mycobacterium paratuberculosis and Mycobacterium avium subsp. silvaticum. Analytical Biochemistry 262, 325-30.
47. Fredd, S. (1995). Standards of approval of new drugs for IBD. Inflammatory Bowel Diseases 1, 284-94.